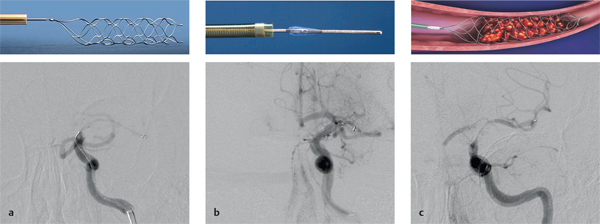
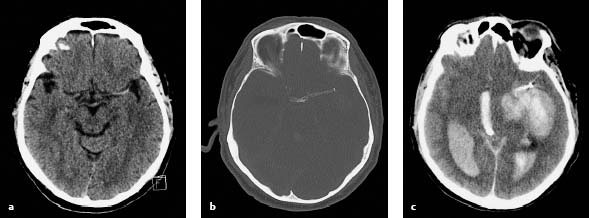
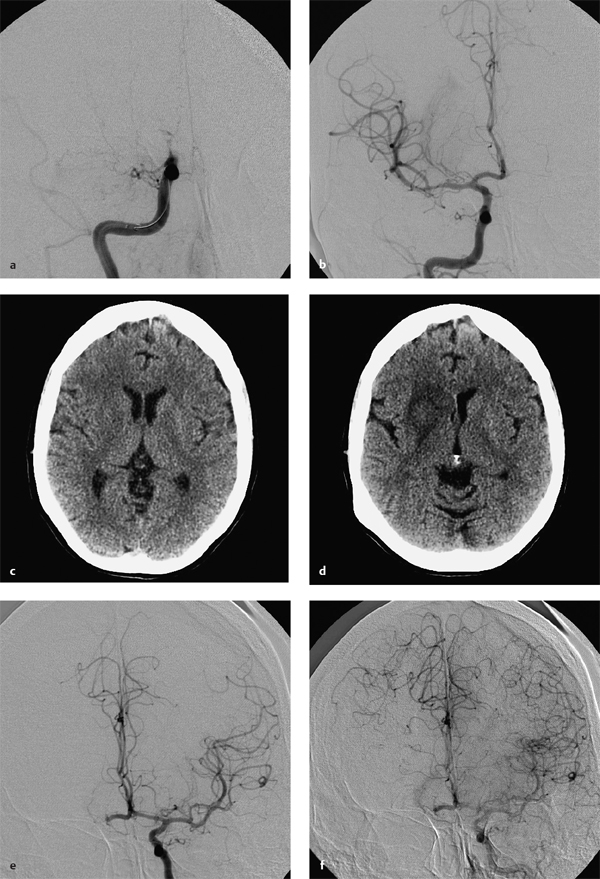
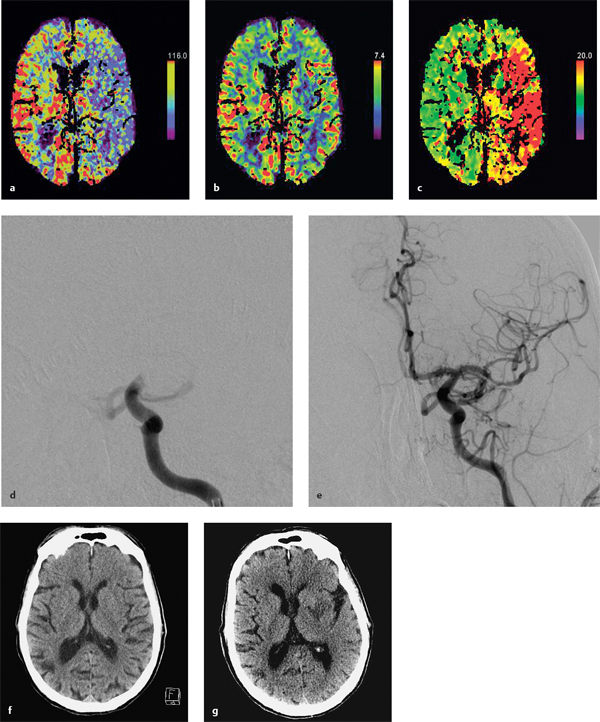
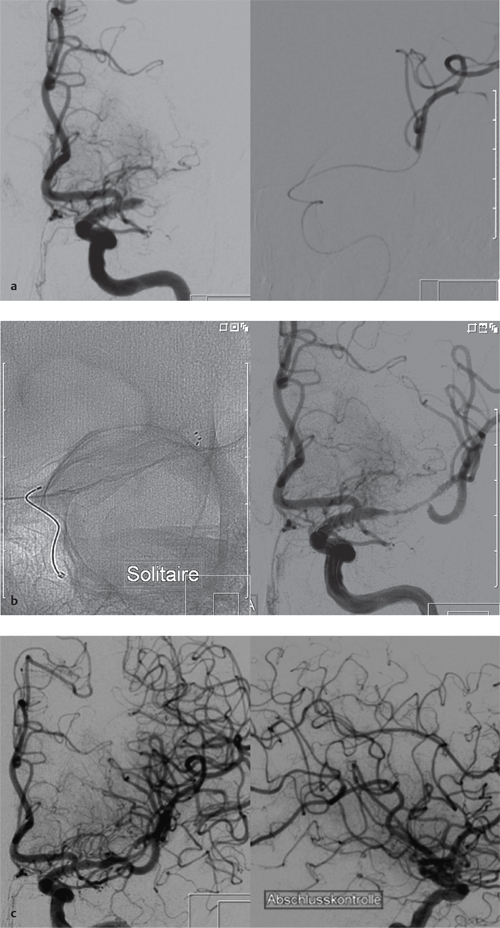
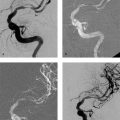
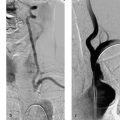
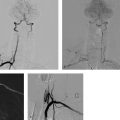
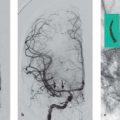
8 Treatment Concepts and Results The intracranial bifurcation of the internal carotid artery (ICA), called the carotid terminus (carotid T), plays a central role in the blood supply to the brain. It is the site where the ICA divides into the middle cerebral artery (MCA) and anterior cerebral artery (ACA), which are the main suppliers of one cerebral hemisphere. As part of the circle of Willis, the carotid T is also critically involved in the collateral blood supply to the hemispheres. An acute occlusion of the carotid T is most often caused by an embolus of cardiac origin. Less frequent causes are arterioarterial emboli arising from a proximal stenosis or atherosclerotic plaque. Generally these thrombi extend from the supraclinoid portion of the carotid siphon into the proximal portions of the MCA (M1 segment) and ACA (A1 segment), blocking antegrade blood flow to the peripheral branches of those arteries. Lenticulostriate perforators from the M1 segment, central branches of A1 (e.g., the medial striate artery), the anterior choroidal artery, and the posterior communicating branch may be obstructed, depending on the extent of the thrombus. Adequate collateral circulation may be maintained through leptomeningeal branches of the ACA or through choroidal and leptomeningeal branches of the posterior cerebral artery (PCA), but this is uncommon. Most acute occlusions of the carotid T cause an extensive infarction involving the basal ganglia, internal capsule, and large portions of the frontal, temporal, and parietal lobes. This results in a very severe, acute hemispheric neurologic syndrome with permanent disability. Carotid-T occlusions have a high mortality rate (Jansen et al. 1995). Note Acute carotid-T occlusions typically cause a major stroke with high morbidity and mortality. The prime therapeutic goal is to restore flow through the occluded vessel as quickly as possible to salvage tissue that is still structurally intact (”time is brain”). An acute cerebrovascular occlusion should be treated without delay. The goal of prompt recanalization is to salvage brain tissue that has already sustained a degree of functional damage but is still structurally intact (penumbra). Treatment options during the early postocclusion period are pharmacologic thrombolysis and mechanical recanalization techniques. Intravenous thrombolysis was approved by the U.S. Food and Drug Administration (FDA) in 1996 for the treatment of acute stroke. This approval is based on the study results of the National Institute of Neurologic Disorders (NINDS) and the rtPA Stroke Study Group (National Institute of Neurologic Disorders and Stroke rtPA Stroke Study Group 1995). Since then, intravenous thrombolysis has become established as the standard treatment for stroke within the first 3 h after symptom onset. Based on the results of the ECASS III study, it was later suggested that the time window for intravenous thrombolysis be expanded from 3 h to 4.5 h (Hacke et al. 2008b). A major advantage of intravenous thrombolysis is its technical simplicity, which allows for rapid initiation of treatment in stroke victims. It does not require complex technology or special interventional training like that needed for mechanical recanalization, for example. In this therapy (see p. 66) the fibrinolytic agent is delivered directly to the site of the thrombus through a microcatheter. This allows a much higher local concentration of the fibrinolytic agent to be delivered to the clot than is possible with intravenous thrombolysis. This selective application also allows the total dose of the fibrinolytic agent to be reduced, thereby lessening the risk of intracranial hemorrhage. In turn, this lower hemorrhage risk allows the therapeutic window to be expanded past the time frame recommended for intravenous thrombolysis (see PROACT I and II trials; del Zoppo et al. 1998; Furlan et al. 1999). The principal agent used for intravenous and intra-arterial thrombolysis is rtPA (alteplase). Urokinase is less commonly used. Mechanical, catheter-based recanalization techniques (see Chapter 7) permit the rapid and safe restoration of blood flow through an acutely occluded vessel. They can even be used in patients who are not eligible for thrombolysis because of contraindications, or have shown poor response to primary thrombolysis. The therapeutic time window for mechanical recanalization alone is considerably longer than that for classic intravenous thrombolysis because the risk of bleeding into a possible infarcted area is lower when thrombolytic agents are not used. For example, patients enrolled in the MERCI and Penumbra trials were treated up to 8 h after onset of clinical symptoms (Smith et al. 2005, 2008; Penumbra Pivotal Stroke Trial Investigators 2009). Of course, mechanical recanalization is more costly and complex than intravenous thrombolysis. It is an interventional procedure that requires proper training and equipment and carries the risks of a cerebral catheter angiography. Interventional risks include vascular dissection, perforation, and vascular occlusion. The development of recanalization techniques is proceeding at a rapid pace. Below we review the main functional principles of devices currently available. Some procedures combine several of the techniques described, and readers are referred to specific sections in this book for technical details. Aspiration systems (see also p. 84) remove the thrombus by suction. The simplest variant is manual aspiration with a large syringe on an aspiration catheter. The ICA can be occluded proximally with a balloon cuff during the aspiration maneuver. More sophisticated systems utilize the Venturi-Bernoulli effect to fragment and aspirate the clot with a saline jet. A widely used system employs a ”separator” wire to fragment the clot and simultaneously aspirate the fragments with a pump connected to the guide catheter (Fig. 8.1 b). Thrombectomy systems are designed to retrieve the thrombus in one piece. The device may use a snare or prongs to grasp the clot from the proximal side and pull it into the guide catheter, or it may capture the clot from the distal side with a brush, corkscrew wire, mesh, or small basket (Fig. 8.1c). Intermittent balloon occlusion of the ICA with a cuffed guide catheter, for example, can prevent clot fragments from embolizing to more distal cerebral vessels. Fragmentation devices break the clot into small pieces. This treatment is usually supplemented by intra-arterial thrombolysis. Microwires, snares, and soft balloons for peripheral transluminal angioplasty are simple types of fragmentation device. More complex systems employ ultrasound or other mechanisms for clot disruption. Intracranial stents are designed to provide rapid restoration of antegrade blood flow. Stent placement also increases the surface area of the clot that is accessible to thrombolysis, enabling the clot to be dissolved more quickly by the endogenous plasminogen-plasmin system or by adjuvant thrombolytic therapy. Fig. 8.1a–c Various recanalization devices in current use. Digital subtraction angiography (DSA) “snapshots” of devices deployed in three patients with an acute carotid-T occlusion. a Solitaire stent (with kind permission of ev3 GmbH, Bonn, Germany). b Penumbra System (with kind permission of Penumbra Europe GmbH, Berlin, Germany). c Bonnet device (with kind permission of Phenox GmbH, Bochum,Germany, http://www.phenox.info). Fig. 8.2a–c A 70-year-old man who presented with left hemispheric syndrome of 4 h duration. a Initial CT shows hyperdensity of the left MCA with no evidence of early ischemic changes. b After several failed thrombectomy attempts using various devices, an intracranial stent was deployed. The stent extends from the distal left ICA into the MCA bifurcation. c Tirofiban (Aggrastat) was administered. Cranial CT the next day shows a large parenchymal hemorrhage with associated mass effect and ventricular invasion. Caution Whenever possible, avoid using stents that are not retrievable from the vessel after deployment. These permanent stents require additional treatment with an antiplatelet drug (e.g., tirofiban), which increases the risk of intracranial hemorrhage (Fig. 8.2). The latest generation of closed-cell stents allow the device to be retrieved after recanalization. Antiplatelet therapy is unnecessary when a temporary stent is used. It has also been found that retractable stents can be successfully used for thrombus extraction. New stentlike systems combine the rapid restoration of antegrade blood flow (temporary bypass function) with the capability for thrombus extraction (Figs. 8.1a, 8.3, and 8.4). Increasingly, multimodal treatment protocols are being instituted at many centers. Different recanalization methods are combined to increase the speed and efficacy of recanalization. Various combinations are possible: intravenous and intra-arterial thrombolysis, intravenous thrombolysis followed by mechanical recanalization, mechanical recanalization accompanied by intra-arterial thrombolysis, or a combination of all three modalities. At our institution, patients with an acute carotid-T occlusion receive immediate intravenous thrombolysis after CT scanning, provided they present within the 4.5-h time window and there are no contraindications to lytic therapy. While continuous intravenous thrombolysis is maintained, the patients are intubated and undergo diagnostic angiography. As a rule, intravenous thrombolysis is almost complete by the time the angiograms are available. In the best-case scenario, patency has been restored to the carotid T; however, if the occlusion persists, mechanical recanalization can be performed without further loss of time. Patients seen initially at a hospital without interventional neuroradiology can be diagnosed and then bridged with intravenous thrombolysis during transport to a stroke center after contraindications have been excluded (see also the section on Bridging Therapy, p. 76). In this way the time to mechanical recanalization is utilized for intravenous thrombolysis, and the clot has already been ”softened” or partially dissolved. Intra-arterial thrombolysis may be done as an adjunct to mechanical recanalization. As noted above, this is an option when retrievable stents are used. The deployed stent restores antegrade blood flow and increases the surface area available for attack by the thrombolytic agent. In turn, the thrombolysis softens the clot and facilitates its extraction. Practical Tip Various catheter systems can be used successively during mechanical recanalization. Not all devices are equally suitable for different situations, so it may be advantageous to change materials during recanalization to achieve a better result. Fig. 8.3a–f A 66-year-old woman who presented with an acute right hemispheric syndrome of 3 h duration. a DSA image before mechanical recanalization of the carotid. T. The right ICA is still occluded following intravenous thrombolysis. b DSA image of the right ICA after mechanical recanalization of the carotid T. Complete thrombectomy with a Solitaire stent has restored perfusion to all MCA and ACA branches on the right side. c Initial cranial CT before recanalization shows no early ischemic changes. d CT scan 2 days after recanalization shows infarction confined to the right basal ganglia. e DSA of the left ICA before recanalization shows definite crossflow through the anterior communicating branch to the right side. f Excellent collateral supply to the right MCA territory has contributed to a good clinical outcome (mRS = 2) in this patient. Fig. 8.4a–g A 78-year-old man who presented 2.5 h after onset of severe aphasia and severe right hemiparesis. a CT perfusion imaging (CTP) shows markedly decreased blood flow in the left MCA territory. b CTP shows a mismatch relative to a with a small area of decreased blood volume in the left MCA territory. c CTP shows significant delay of contrast medium transit time throughout the left MCA territory with normal perfusion in the left ACA territory and good collateral flow through the anterior communicating branch (a–c). d DSA of the left ICA before mechanical recanalization of the carotid. T. After completion of intravenous thrombolysis, the left ICA is still occluded above the origin of the posterior communicating branch. e DSA after mechanical recanalization with a Solitaire stent shows normal filling of the MCA and ACA branches on the left side. f Cranial CT shows no early ischemic changes prior to the intervention. g CT scan 1 day after recanalization shows small infarction confined to the left basal ganglia. Two main criteria are used to evaluate the efficacy of different treatment methods for acute carotid-T occlusions: recanalization rates and clinical results. Several studies have confirmed that acute carotid-T occlusions do not respond well to pharmacologic thrombolysis alone. Saqqur et al. (2007) found that intravenous thrombolysis produced complete recanalization of the carotid T by Doppler ultrasound in only 6% of patients treated within the 3-h window. Jansen et al. (1995) reported recanalization rates of only 12.5% after intravenous or intra-arterial thrombolysis. Arnold et al. (2003) found partial recanalization of the ICA in 63% of patients after intra-arterial urokinase, but this treatment restored MCA patency in only 17%. In a comparative study on the efficacy of intra-arterial thrombolysis in different types of occlusion, Eckert et al. (2003) reported a recanalization rate of 23% for carotid-T occlusions, compared with a 70% rate for M1 occlusions of the MCA. Zaidat et al. (2002) described a recanalization rate of 50% for carotid-T occlusions that were treated by intra-arterial thrombolysis or a combination of intravenous plus intra-arterial thrombolysis. Significantly better recanalization rates for carotid-T occlusions have been reported with mechanical recanalization techniques alone or combined with thrombolysis. For example, the Multi MERCI trial described a recanalization rate of 65% for carotid-T occlusions (Smith et al. 2008). The RECANALISE study noted a definite advantage of combining intravenous thrombolysis with endovascular therapy compared with intravenous thrombolysis alone. In the patient group with an ICA occlusion or combined ICA and MCA occlusion, the combination of intravenous thrombolysis and endovascular therapy yielded a recanalization rate of 69%, vs. only 30% with intravenous thrombolysis alone (Mazighi et al. 2009). Lin et al. (2009) published their results in a series of 75 patients with carotid-T occlusions treated by intra-arterial thrombolysis, the MERCI device, stents, or a combination of these methods. The best recanalization rates (86%) were achieved using a combination of intra-arterial thrombolysis and the MERCI device. This contrasted with a recanalization rate of only 46% after use of the MERCI device alone and 18% after intra-arterial thrombolysis. In our study, consisting of 14 patients with acute carotid-T occlusions, various mechanical recanalization techniques were used alone and in conjunction with pharmacologic thrombolysis. The combined approach restored ICA patency in 93% of cases and recanalized the carotid T and MCA (M1 segment and at least one M2 branch) in 79% of cases (Fesl et al. 2011). Note Multimodal therapy not only improves recanalization rates but can also shorten the time to recanalization. This principle is illustrated by a post-hoc analysis of the MERCI and Multi MERCI trials (Shi et al. 2010), which showed a definite trend toward shorter intervention times in patients that received previous intravenous thrombolysis. Several studies have shown that pharmacologic thrombolysis alone does not yield acceptable clinical results. Arnold et al. (2003) found that only 17% of patients achieved a good outcome (mRS ≤ 2) after intra-arterial urokinase therapy alone, with a mortality rate of 42% after 90 days. Eckert et al. (2003) reported a poor clinical outcome in 49% of patients with carotid-T occlusions (Barthel index < 50) and a mortality rate of 43%. Jansen et al. (1995) found a good or moderate outcome (mRS = 1–3) in 16% of patients after intravenous or intra-arterial thrombolysis, with a mortality rate of 53%. Zaidat et al. (2002) described a mortality rate of 70% in patients with acute carotid-T occlusions who had received thrombolytic therapy. Mechanical recanalization alone and combinations of mechanical thrombectomy with thrombolysis provide very good recanalization rates, but the clinical outcomes often fail to meet expectations. In the Multi MERCI trial, for example, 33% of patients with carotid-T occlusions had a good clinical outcome (mRS ≤ 2) with a mortality rate of 45% (Smith et al. 2008). Lin et al. (2009) described a favorable outcome in 23% of patients with a carotid-T occlusion. The mortality rate in this study was 37%. In the Penumbra study (Penumbra Pivotal Stroke Trial Investigators 2009), 23% of the patients achieved a good or satisfactory outcome (mRS ≤ 3), and the mortality rate at 90 days was 57%. Our results are comparable, with a good clinical outcome in 21% of patients and a mortality rate of 43% (Fesl et al. 2011). Note All recanalization studies support the concept that a good clinical outcome depends critically upon whether the acute occlusion could be recanalized. A large meta-analysis of 53 studies in 2066 patients with acute vascular occlusions describes successful recanalization as the most important predictor of a good clinical outcome (Rha and Saver 2007). An analysis of the MERCI, Multi MERCI, and RECANALISE studies also indicates that patients with successful recanalization achieve significantly better clinical results (Mazighi et al. 2009; Shi et al. 2010). The time from symptom onset to recanalization also affects clinical outcomes. In the RECANALISE study, for example, patients who had the shortest times to recanalization with a combination of intravenous thrombolysis and endovascular therapy had the best outcomes (Mazighi et al. 2009). A study of pooled data from the MERCI trials found an association between short intervention time and a lower mortality rate at 90 days (Shi et al. 2010). Most acute carotid-T occlusions are poorly collateralized and cause extensive infarctions with high morbidity and mortality. The key to successful treatment lies in shortening the time from symptom onset to recanalization and restoring flow before a large infarction can develop. Pharmacologic thrombolysis alone generally does not provide effective recanalization or a satisfactory clinical outcome. As a result, acute carotid-T occlusions should be reopened by mechanical recanalization as quickly as possible. Prior intravenous thrombolysis or concomitant intra-arterial thrombolysis may support successful recanalization and shorten the time to flow restoration. Practical Tip If there are no contraindications to thrombolysis, we recommend a combination of intravenous thrombolysis and endovascular therapy. Intravenous thrombolysis can be started immediately after the clinical and CT examination and continued while the intervention is prepared. If mechanical recanalization is not available on site, then the patient, while still on thrombolysis, should be transferred to a neurovascular center where endovascular recanalization can be performed without further loss of time. The current trend in mechanical recanalization is toward the use of temporary stents, which permit the very rapid and safe recanalization of carotid-T occlusions. Despite the high recanalization rates, the clinical outcomes of acute carotid-T occlusions are often unsatisfactory. This is due partly to the central location of the occlusion combined with a usually poor collateral supply, creating an enormous time pressure for initiating treatment. Another crucial factor is patient selection. For example, a patient’s age and initial clinical status will greatly influence the clinical outcome of recanalization. Besides the technical advancement of recanalization therapies, future research should focus on identifying clinical and imaging criteria that will enable better patient selection. Controlled, randomized multicenter studies such as the Interventional Management of Stroke Study III (IMS III) now under way will certainly make a valuable contribution to optimizing the acute management of stroke (Khatri et al. 2008). Two-thirds of all ischemic strokes occur in the anterior circulation, and these strokes commonly involve the MCA. The occlusion may be confined to the MCA or may coexist with a carotid-T occlusion described in the previous section (p. 103). Unlike the symptoms of decreased blood flow in the posterior circulation, which are variable and often take a gradually progressive course since they often result from a preexisting stenosis, most anterior circulation strokes are caused by a sudden thromboembolic vascular occlusion from a cardiac or arterioarterial source. The embolus plugs the vessel abruptly like a cork in a bottle and may continue to move, depending on its size and malleability. Agglutination clots may additionally form due to the proximal or distal stagnation of flow. The severity of clinical manifestations depends on the thrombus burden, the site of the occlusion, and individual angioarchitecture. The last of these factors will determine the involvement of the noncollateralized perforators, which supply the basal ganglia and internal capsule, as well as the degree of collateral flow from nonoccluded vascular territories. These circumstances critically define the factors that will determine the success or failure of conservative intravenous or interventional treatment techniques. Note Thrombus burden, thrombus location, and individual angioarchitecture are key factors determining the clinical severity of a stroke. Because an accurate description of the occlusion pattern is necessary in daily communications between clinical colleagues and in the interpretation of study results, and because that pattern will dictate clinical presentation and therapeutic strategies, especially with MCA occlusions, it is important to review briefly the anatomy of the middle cerebral artery (see also the section on Segmental Anatomy of the MCA, p. 8). The MCA is typically divided into four segments: • M1: sphenoidal • M2: insular • M3: opercular • M4: multiple cortical segments This classification is based upon classic, systematic neurosurgical analyses of vascular variants. Initial publications on MCA segmental anatomy date back to the late 1930s (Agarwal et al. 2004) and based the nomenclature on the relationship of the vessel to anatomic landmarks, rather than on hemodynamics. Moreover, the great variability of the MCA bifurcation (e.g., it may form a trifurcation or a large temporal branch with a far proximal origin) has long been recognized, but is sometimes ignored in everyday practice and even in studies. Especially in distinguishing the M1 and M2 segments, it is widely assumed that the M1 segment terminates at its bifurcation. In fact, however, this classification defines one prebifurcation segment and two or more postbifurcation M1 segments, which become M2 only after passing the limen insulae and entering the sylvian fissure. As noted above, involvement of the lenticulostriate perforators, which are end arteries without a collateral supply, has a major bearing on clinical outcome and on the time frame within which recanalization can positively affect the outcome. Like the main trunks of the MCA itself, however, the perforators are also subject to anatomic variations, both in their number and in their origin relative to, say, the M1 bifurcation. Given this variability, a proximal M1 occlusion in one patient may have relatively mild symptoms or at least a favorable clinical outcome even without successful recanalization, owing to adequate retrograde flow from lenticulostriate perforators arising at far lateral sites. Meanwhile, a different patient may suffer an infarction or hemorrhage despite early recanalization because all the perforators arise from the occluded segment. Since studies have shown that perforator infarctions are likely to bleed in response to systemic thrombolysis, radiologically detected involvement of the area supplied by the perforators (the basal ganglia) would provide a strong rationale for mechanical recanalization. In a study by Vora et al. (2007) of 185 multimodal stroke treatments, this rationale is supported by the fact that 51% of patients with M1 occlusions showed hemorrhagic imbibition of the infarcted area and 42% had a parenchymal hemorrhage. The figures in patients with M2 occlusions were only 7% and 4%, respectively. Hemorrhages were particularly common in cases where multimodal therapy included intravenous or combined intravenous plus intra-arterial fibrinolysis. Caution Involvement of the lenticulostriate perforators means a greater risk of hemorrhage in response to thrombolysis and recanalization! For the same reason, an important goal of mechanical recanalization is to reopen the perforator-bearing segment—usually the proximal M1 segment—as quickly as possible. When a thrombus is manipulated by balloon angioplasty or stenting, care should be taken that the clot moves away from the perforators, which usually arise posterosuperiorly, to avoid the prolonged occlusion of these small vessels, which can no longer be catheterized during the rest of the intervention. Practical Tip Early reperfusion of the perforators is an important goal in mechanical recanalization. The placement of a stent or balloon should displace the thrombus in a downward and anterior direction, away from the perforator origins. A systematic study of angiographic occlusion patterns and NIHSS scores in over 200 patients by a group of authors in Bern, Switzerland (Fischer et al. 2005) clearly documented the strong correlation that exists between the location of a vascular occlusion and the severity of the resulting stroke, despite possible anatomic variants. In patients with an NIHSS score > 12, the positive predictive value for detecting a trunk occlusion, considered here to include the M1 segment, was > 90%. Note In more than 90% of cases, a severe stroke with NIHSS > 12 results from an occlusion of the MCA trunk with involvement of the Ml segment. For a review of the imaging features of the various occlusion patterns in stroke, we refer the reader to the corresponding sections in Part I of this book. The technical aspects of MRI and CT examinations are covered in recommendations published in 2008 (Wintermark et al. 2008). Although MRI is unquestionably the more versatile modality, the majority of studies dealing with stroke therapy are still based on CT. The protocol for both modalities, however, should include vascular imaging and the quantitative assessment of perfusion. Without going into too much detail, current evidence suggests that perfusion is important in predicting both the efficacy of recanalization by systemic thrombolysis and the likelihood of hemorrhagic transformation, at least in patients with an MCA occlusion. For example, in a multivariate analysis of numerous factors, Gupta et al. (2006) found that only a decrease in cerebral blood flow to < 13 mL 100 g−1 min−1 was associated with a significantly increased likelihood of intracranial hemorrhage in patients who received intra-arterial thrombolysis (odds ratio [OR] 1.58, acquisition with xenon CT). Another analysis of these data showed that perfusion maintained at a level > 20 mL 100 g−1 min−1 was associated with a significantly greater likelihood of successful thrombolysis (Jovin et al. 2007). These discoveries have definite therapeutic implications. As the treatment modalities for stroke continue to advance, these insights could help to identify patients who would likely benefit from pharmacologic therapy and those who would benefit from primary mechanical recanalization. Practical Tip Perfusion in the infarcted area correlates not only with survival of the penumbra but also with the risk of hemorrhage and the likelihood of successful thrombolysis. Whenever possible, therefore, the CT or MRI examination should include perfusion imaging. Major studies on intravenous fibrinolysis with rtPA have been mentioned throughout this book. For MCA occlusions too, intravenous thrombolysis up to 4.5 h after symptom onset is considered the current treatment standard according to the NINDS (National Institute of Neurological Disorders and Stroke rtPA Stroke Study Group 1995) and ECASS III trials (Hacke et al. 2008b). Details may be found in the sections dealing with thrombolysis (see Chapter 6). The fact that intravenous rtPA has very limited effectiveness for MCA trunk occlusions, presumably depending on the thrombus burden and occlusion pattern in NINDS (which implies the presence of a trunk occlusion only on the basis of stroke severity), is also noted earlier in the book (see the section on Intravenous Thrombolysis, p. 52). Earlier experience on the intra-arterial use of fibrinolytic agents (Hacke et al. 1988) for basilar artery occlusions already demonstrated the theoretical advantages of this procedure, which can directly visualize the occlusion while also delivering significantly higher concentrations of the fibrinolytic agent to the targeted site. The possibility of combining local intra-arterial fibrinolysis with mechanical manipulation of the thrombus is explored later. It is self-evident that all intra-arterial therapies are more costly and complex because of their invasiveness, the need for specialized training and equipment, and potential complications relating to puncture site, catheter insertion, catheterization of the cerebral arteries, and the use of general anesthesia at some centers. These two studies, published in 1998 and 1999 (del Zoppo et al. 1998; Furlan et al. 1999; see also Table 6.2), dealt with the intra-arterial use of prourokinase in patients with an angiographically detected M1 or M2 occlusion. PROACT I was designed to investigate the safety and efficacy of prourokinase. In that study, two groups of 40 patients each received 6 mg of prourokinase or placebo over a 2-h period from 3 to 6 h after onset of stroke symptoms. The agent had to be administered proximal to the thrombus; manipulation with a wire or catheter was not allowed. The TIMI (Thrombolysis in Myocardial Infarction) score (Table 8.1) was used to evaluate recanalization. This classification is derived from studies on thrombolysis in acute myocardial infarction, and an original or slightly modified form of the classification is still used in studies today. In the PROACT I trial, at least partial reperfusion (TIMI = 2 or 3) was demonstrable in 57.7% of the patients vs. only 14.3% of the controls. Although the rate of symptomatic intracranial hemorrhage was more than twice as high in the treated group (15.5%) than in the control group (7.1%), the patients treated with intra-arterial prourokinase had a better clinical outcome and lower mortality. While these advantages were very promising but not statistically significant due to the small case numbers, PROACT II studied 180 patients using the same design except for a higher administered dose (9 mg). Of the 180 patients enrolled, 121 received prourokinase plus intravenous heparin; 59 patients served as a control group and underwent arterial catheterization but received only a saline infusion through the microcatheter plus intravenous heparin. The physicians conducting the study were blinded to the group assignments. As expected, the partial and complete recanalization rates were significantly higher in the prourokinase group (66%) than in the control group (18%). This benefit was also reflected in the percentage of patients who survived and were functionally independent (40% vs. 25%). The higher incidence of intracranial hemorrhage (10% vs. 2% in the control group) did not affect the mortality rate at 90 days, which was 25% compared with 27% in the control group. Despite this obvious benefit, the FDA withheld approval of prourokinase for use in acute stroke pending the results of further studies. Tab. 8.1 Thrombolysis in Myocardial Infarction (TIMI) score for classifying perfusion after the recanalization of a thromboembolic occlusion (TICI classification see Table 7.3) TIMI score Description 0 No perfusion, no antegrade flow distal to the occlusion 1 Penetration of the thrombus with minimal distal flow; filling around the thrombus but no distal branch filling 2 Partial perfusion with incomplete or slow distal branch filling 3 Full perfusion with timely filling of arteries and veins Source: The TIMI Study Group 1985. Since PROACT I and II, many studies have been published on local intra-arterial fibrinolysis, some even reporting better reperfusion results with no increased incidence of intracranial hemorrhage. A comparative study of intravenous and intra-arterial thrombolysis between two stroke units in Switzerland published in 2008 again documented the benefit of local fibrinolysis in ischemic stroke patients with a HMCAS (Mattle et al. 2008). With a population of > 50 patients per unit, the group that received local intra-arterial fibrinolysis had > 30% more independently surviving patients than the group that underwent intravenous thrombolysis at the other unit. This is all the more remarkable when we note that baseline stroke severity was higher in the group treated with local intra-arterial fibrinolysis, and that the mean time to treatment was considerably longer in the intra-arterial group (244 min) than in the intravenous group (156 min). Intravenous thrombolysis can be administered at a reduced dose to bridge the time until the initiation of local fibrinolysis. Bridging therapy is described in an earlier chapter (see p. 76) and does not apply exclusively to MCA occlusions. A major disadvantage of intravenous as well as intra-arterial fibrinolysis without mechanical manipulation is that, with a complete occlusion, the fibrinolytic agent can interact with the thrombus only to a very limited degree. Thus, it was recognized very early that supportive manipulations could increase the recanalization rate. When the studies on intravenous thrombolysis were published in the 1990s, initial reports appeared on how simple microwire manipulations during fibrinolysis could support and improve recanalization. Even today, this can still be an effective tool in settings which lack availability or experience with approved mechanical thrombectomy systems. As recently as 2008, a study was published in which 18 of 19 patients were successfully recanalized by urokinase therapy that was aided by microwire manipulation (Kim et al. 2008). Note Mechanical manipulations support the effectiveness of fibrinolysis. Ultrasound provides a noninvasive mechanical option for supporting fibrinolysis. Similar to its use in cleaning surfaces, ultrasound energy can induce the mixing and movement of fluids without causing grossly visible flow (see also the section on Classification of Symptoms, p. 97). Ultrasound was found to accelerate fibrinolysis in experimental models (Francis and Suchkova 2001). With isolated reports of continuous transcranial Doppler ultrasound improving the efficacy of intravenous thrombolysis, leading to better clinical outcomes, this phenomenon was subsequently investigated in studies. The most important of these studies, CLOTBUST (Alexandrov et al. 2004), is relevant in that it dealt exclusively with occlusions of the MCA. A total of 126 patients were assigned to two equal groups that received either continuous 2-MHz transcranial Doppler ultrasound at the occlusion interface (target group) or a placebo (control group) during continuous intravenous thrombolysis. Both the rate of successful recanalizations and the percentage of patients with little or no disability were higher in the target group than in the control group. Statistical analysis of the results did not indicate a significant trend, however. This concept can be expanded to include the concurrent use of ultrasound contrast agents (microbubbles) to amplify the disruptive effect of the ultrasound. Study results have not yet been published on this refinement. The intra-arterial counterpart of ultrasound-enhanced thrombolysis involves the use of intravascular ultrasound or the EKOS catheter system (see Fig. 7.8), which have been used for example in the IMS II and III trials (IMS II Trial Investigators 2007; Interventional Management of Stroke III: intravenous thrombolysis vs. intravenous plus local intra-arterial thrombolysis [bridging]; patients recruited since June 2006). Again, ultrasound energy supports the interaction of the fibrinolytic agent with the clot but is delivered through the tip of the microcatheter itself. Traditional treatment methods have been based wholly or partly on the action of fibrinolytic agents, with or without mechanical support. A large percentage of patients are ineligible for lytic therapy because of possible contraindications or because the time from symptom onset is too long or unknown. Today, devices and systems for primary thrombus extraction have attracted growing interest and are the subject of numerous studies. Because these devices are not specific for thrombus location and are described earlier in some detail, we limit our attention here to a brief review. The MERCI Retriever is the first thrombectomy system to be approved by the FDA for use in stroke patients (see also the section on Recanalization Rate and Neurologic Outcome, p. 89). The first generation of this device, which is deployed distal to the clot through a microcatheter and has a corkscrew-like shape, was investigated for its safety and efficacy in a total of 151 patients, 57% of whom had an occlusion of the MCA. The rate of successful recanalizations, at 46% (intention to treat), was not very satisfactory, and the incidence of significant complications, at 7.1%, was unacceptably high from a current perspective. The third-generation MERCI Retriever (V Series) is now being marketed with a design that differs significantly from its predecessors. With regard to MCA occlusions, it is noteworthy that the smallest retriever (2 mm) can even reach the M2 segment. No study results have yet been published on the new design. Based on data in the MERCI Registry, the device has an overall revascularization rate of ~80%. This modular system (see Fig. 7.10) consists of a brushlike array of nylon microfilaments radiating from a central wire, which may or may not be combined with a nitinol microcage. It is important in the treatment of MCA occlusions because the smallest version can be deployed in vessels 1–1.5 mm in diameter and, at least in theory, can recanalize occlusions at the M3 level. No clinical studies or comprehensive series have yet been published. Stent-based thrombectomy devices are a relatively new and promising approach now being investigated in clinical studies and produced by several manufacturers (see Stenting in Stroke Patients, p. 92). The main goal is not to insert a stent to restore flow but to use its mechanical interaction with the thrombus as a means of extracting the clot. Initial reports and single-center studies have shown that this concept can provide high recanalization rates with few complications (Castaño et al. 2010). Efficacy and safety both depend critically on the mechanical properties of the device such as mesh size, radial pressure, friction during placement, and the surface characteristics of the material, which are currently varied and optimized by various manufacturing firms. At present, the only systems that have received CE (European Community) approval are the Solitaire FR (EV3) and Trevo device (Concentric Medical). It should be added, however, that because these systems also have a temporary endovascular bypass function and are used after or during the administration of fibrinolytic drugs, their success is not based entirely on mechanical clot retrieval, as explained below. This concept seeks to recanalize the occluded artery as quickly as possible to provide immediate flow restoration to dependent areas, allow local or systemic fibrinolytic agents to reach the thrombus, and clear prothrombogenic substances formed by the clot. Kelly et al. (2008) described a patient with an embolic occlusion of the M1 segment of the right MCA and an associated occlusion of the right cervical ICA that precluded thrombectomy with the MERCI device or other approved system. As an alternative, the authors introduced a microcatheter through the contralateral ICA and advanced it across the anterior communicating artery and through the thrombus in the right MCA. The microcatheter was withdrawn to partially deploy a self-expanding, closed-cell nitinol stent (Enterprise, Codman) across the occlusion site. Two-thirds of the stent was unconstrained to circumferentially displace the thrombus and restore blood flow through the right MCA. Abciximab and rtPA were administered intra-arterially through the microcatheter to dissolve the clot. The stent was then reconstrained and removed. Note Besides the minimal need for manipulations (thrombectomy was not done in this case), the advantage of a temporary retractable stent over a permanently implanted stent is that it eliminates the need for long-term platelet inhibition. This can be particularly relevant in stroke patients who are already on anticoagulants for an underlying cardiac arrhythmia. While the stent used in this initial description was only partially deployed before retrieval, systems are currently being used that are designed almost exclusively for this application. Besides the Trevo and Solitaire systems described earlier, which are designed primarily for thrombectomy (Fig. 8.5), today there are systems specifically designed to produce an optimum bypass effect (e.g., MindFrame devices). Fig. 8.5a-c Occlusion of the left MCA. a The occlusion is crossed with a microcatheter, and contrast medium is injected distal to the thrombus (right). b The microcatheter is carefully withdrawn to deploy the stent in the occluded segment (left). Contrast injection through the guide catheter (right) shows the efficacy of the temporary endovascular bypass with filling of distal vessels, but with persistent occlusion of lenticulostriate perforators. c After stent retrieval for mechanical thrombectomy, complete perfusion is restored (TIMI score = 3), including the perforators from the M1 segment.
Carotid-T Occlusion
Introduction
Treatment
Intravenous Thrombolysis
Intra-arterial Thrombolysis
Mechanical Recanalization Techniques
Aspiration Systems
Thrombectomy Systems
Fragmentation Devices
Intracranial Stents
Combination Therapies
Study Results
Recanalization Rates
Clinical Results
Conclusion
Occlusion of the Middle Cerebral Artery
Introduction
Anatomy
Imaging Tests
Concepts: Intravenous or Intra-arterial Thrombolysis or Mechanical Extraction?
PROACT I and II Trials
Bridging Therapy
Support of Fibrinolysis
Ultrasound
Mechanical Thrombectomy
MERCI Retriever (Concentric Medical)
Phenox
Thrombectomy with Retractable Stents
Concept of Temporary
Endovascular Bypass
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree