1 Radiofrequency Ablation: Mechanism of Action and Devices
The application of radiofrequency (RF) energy and its thermal effects on tissue were described as early as 1891 by d’Arsonval, when RF waves that passed through tissue were observed to cause an increase in local tissue temperature. RF energy became incorporated into practical medicine via the invention of the Bovie knife used for both cauterization and cutting tissue by varying the RF current. A pulsed current caused cauterization of tissue, whereas a more continuous current caused cutting of tissue. The first-generation Bovie knife was a crude monopolar RF electrode with surface adhesive skin pads closing off the circuit. Use of RF application in thermal ablation was first reported by Rossi and by McGahan et al independently in 1992 for liver tumor ablation.
Since the early RF description, over 100,000 estimated liver RF ablation (RFA) procedures have been performed worldwide. The interest in percutaneous tumor ablation has considerably evolved with introduction of several other thermal modalities, including microwave, cryoablation, high-intensity ultrasound, irreversible electroporation, and interstitial laser.1 The evidence thus far suggests, however, that RFA should remain the prototypical ablation device, particularly for lesions <3 cm, and should still be the cornerstone of any ablation practice.2,3 It is the most frequently used ablative technique, and it has the longest track record.4 Familiarity with the RFA mechanism of action, clinical rationale, and techniques remains critical for the success of an ablation practice.5
♦ Physics and Principles
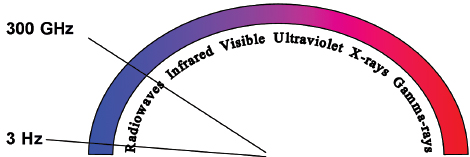
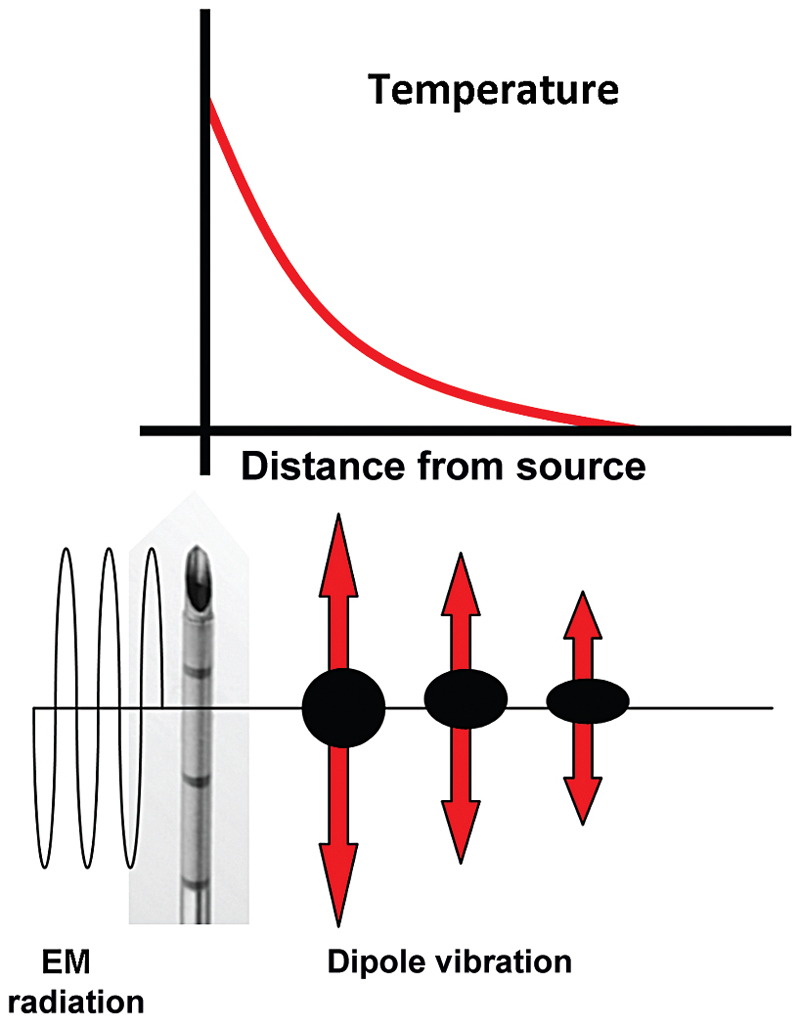
Radiofrequency refers to the part of the electromagnetic (EM) spectrum bounded by the frequencies of 3 Hz and 300 GHz ( Fig. 1.1 ). EM radiation includes (in addition to radio waves) infrared radiation, the visible spectrum, ultraviolet radiation, x-rays, and γ-rays, in increasing frequency.
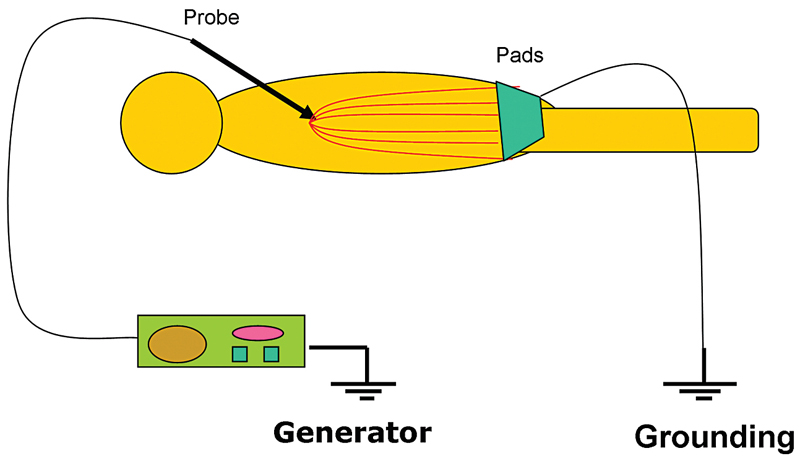
Even though all EM radiation subtypes have the same basic physical properties, their interactions with matter can be very different depending on their frequency and the type of matter. RF waves, as applied in medicine, cause thermal ablation of a defined volume of tissue. The RFA probe acts as the cathode of an electrical circuit that is closed by the application of dispersing pads on the patient’s thighs ( Fig. 1.2 ). Because of the small cross-sectional area of the probe tip, there is a very high energy flux around it. On the other hand, the large cross-sectional area of the grounding pads disperses the energy, minimizing the energy flux. As a result, tissue damage is limited to the part of the circuit that surrounds the probe tip.3
RF ablation results in coagulative necrosis of tissue at high temperatures. The dipole molecules (mostly water) adjacent to the tip of the RF electrode attempt to remain aligned in the direction of current and are forced to vibrate as a rapidly alternating current is applied. Molecules farther away from the probe are set into motion by other vibrating molecules near them. The frictional energy losses between adjacent molecules result in local energy deposition and temperature increase. As one moves away from the source (probe), energy deposition and thus temperature both drop ( Fig. 1.3 ). The RF electrode itself is not the source of heat and is not hot to the touch. It generates an alternating EM field that sets adjacent molecules into motion and intense agitation. The molecules immediately adjacent to the probe are the source of the heat, which is transmitted farther by tissue conductivity.
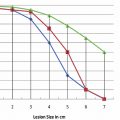
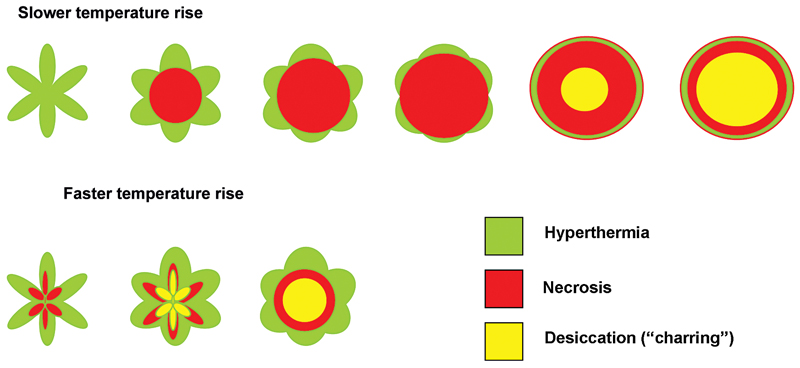
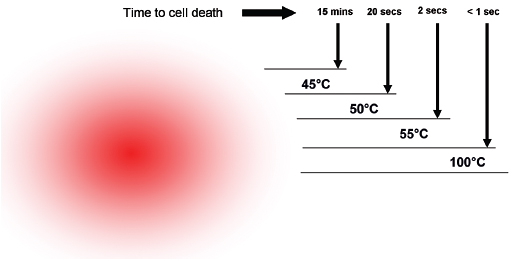
One of the limitations of RFA is that it is heavily dependent on good electrical and thermal tissue conductivity for effective ablation.3 If one pushes the generator’s power too high too quickly, the tissue around the probe becomes desiccated (charred). The desiccated tissue acts as an insulating “sleeve” around the probe, which limits the transmission of further electrical or thermal energy ( Fig. 1.4 ) and limits any further extension of desired tissue destruction. It is instructive to note that time is just as crucial in achieving a large ablation zone as the maximum temperature reached. Figure 1.5 demonstrates the approximate time needed for tissue death at various temperatures. Mammalian tissue is very sensitive to temperature changes. At 55°C, for example, tissue death results within 2 seconds. At 100°C, death is instantaneous as evaporation occurs. Microbubbles are produced and represent gases, primarily nitrogen, that are released from the cells. This, however, is not desired in RFA because of the insulating effect of charred tissue. Thus, a slow, methodical energy deposition is more effective than a quick temperature rise for purposes of enlarging intentional tissue ablation ( Fig. 1.6 ).6 The objective is to heat tissues to 50° to 100°C for 4 to 6 minutes without causing charring or vaporization. If temperatures greater than 105°C are rapidly reached, this causes boiling, vaporization, and carbonization, all of which decrease energy transmission and consequently limit larger ablation sizes.
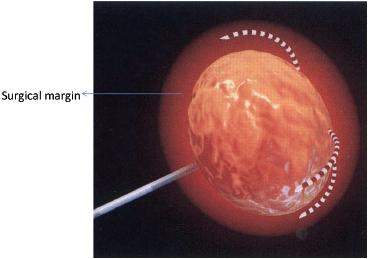
Extrapolated from surgical data, the goal of the RFA technique is to intentionally ablate a zone of healthy tissue around the target tumor, analogous to a “surgical margin” ( Fig. 1.7 ). This margin should be 0.5 to 1.0 cm of ablated normal tissue, based on the difficulty in truly identifying exact tumor margins and the concerns for microscopic tumor extension beyond those confines. That means for a 2-cm tumor, one would need to produce an ablation of approximately 3 to 4 cm.5,6
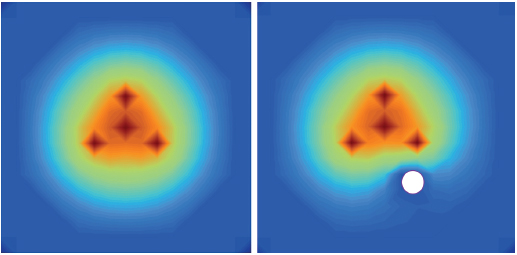
There is considerable heterogeneity of heat deposition through any RFA tissue volume. The extent of coagulation necrosis is dependent on the energy deposited, which is the local tissue interaction minus the heat lost from cooling effects such as the “heat sink” of adjacent blood vessels.5 The heat-sink effect is a phenomenon that limits the effectiveness of all thermal ablation methods. When the target lesion abuts a blood vessel 3 mm or larger, the flowing blood prevents large temperature variations in the part of the tumor near the lesion, thereby keeping the tissue “cooler” ( Fig. 1.8 ). This potentially leaves behind residual unablated tumor near the vessel wall, increasing the chance of local tumor progression. Factors that influence local tumor progression after RFA include tumor-related factors (size, site, orientation, organ, tumor histology, tumor biology) and technical factors (electrodes, generator power, heat sink, time).
Data in RFA of liver primary tumors have demonstrated that 3 cm or less is the optimal size, and there is a persistent effort to improve and enlarge ablation size, through improved RFA techniques. Goldberg and Dupuy6 simplified an approach to the basis of RFA: Induced coagulation necrosis = (energy deposited × local tissue interactions) – heat loss. Investigators have suggested different approaches to solve these physical constraints by modulating tissue characteristics, increasing RF energy deposition, or modifying blood flow.
♦ Generators
Early RF generators for percutaneous application produced modest outputs of 50 W. Today, generators manufactured by the three major RFA companies in the United States are all capable of outputs of 150 to 200 W, delivering high-frequency (460–500 kHz) alternating current via RF electrodes (usually 14- to 17-gauge) of varying configurations. However, the three systems have distinctly different electrode designs and philosophies, varying energy deposition algorithms, and ablation end points. No definitive data have shown one system to be superior to another. Despite the company-suggested algorithms, there is great variability among users. Personal preference and familiarity still play distinct roles in the best and most consistent achievable patient outcomes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree