Neoplasms (malignant) |
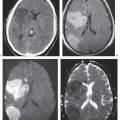
Metastatic tumor |
Single or multiple well-circumscribed or poorly defined infiltrative lesions involving the vertebral marrow, dura, and/or leptomeninges; low to intermediate attenuation; may show contrast enhancement, with or without medullary and cortical bone destruction (radiolucent), with or without bone sclerosis, with or without pathologic vertebral fracture, with or without epidural tumor extension causing compression of neural tissue or vessels. Leptomeningeal tumor often best seen on postcontrast images. |
May have variable destructive or infiltrative changes involving single or multiple sites of involvement. |
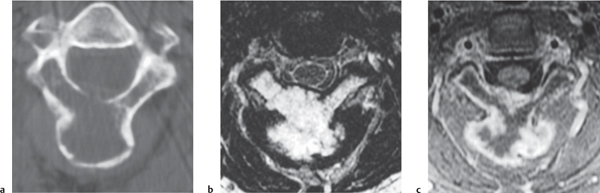
Myeloma/plasmacytoma
Fig. 10.11a, b |
Multiple (myeloma) or single (plasmacytoma), well- circumscribed or poorly defined, diffuse infiltrative radiolucent lesions involving the vertebra (e), and dura; involvement of vertebral body lesions typically radiolucent/bone lysis, rarely involves posterior elements until late stages, low to intermediate attenuation; may show contrast enhancement. Pathologic vertebral fracture, with or without epidural tumor extension causing compression of neural tissue or vessels. |
May have variable destructive or infiltrative changes involving the axial and/or appendicular skeleton. |
Lymphoma and leukemia |
Single or multiple, well-circumscribed or poorly defined, infiltrative radiolucent lesions involving the marrow of the vertebrae, dura, and/or leptomeninges; low to intermediate attenuation, pathologic vertebral fracture, with or without epidural tumor extension causing compression of neural tissue or vessels. May show contrast enhancement, with or without bone destruction. Diffuse involvement of vertebra with Hodgkin lymphoma can produce bone sclerosis, as well as an “ivory vertebra” pattern that has diffuse high attenuation. Leptomeningeal tumor often best seen on postcontrast images. |
May have variable destructive or infiltrative marrow/bony changes involving single or multiple vertebral sites. Lymphoma may extend from paraspinal lymphadenopathy into the spinal bone and adjacent soft tissues within or outside the spinal canal or initially involve only the epidural soft tissues or only the subarachnoid compartment. Can occur at any age (peak incidence third to fifth decades). |
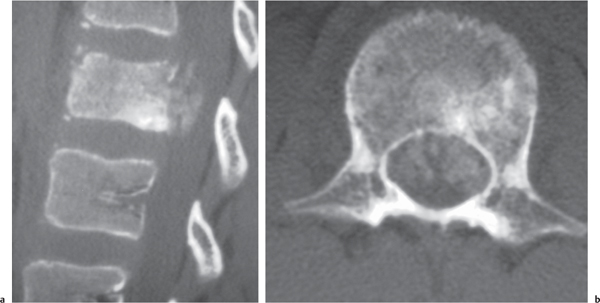
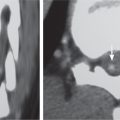
Chordoma
Fig. 10.12a–d |
Well-circumscribed, lobulated radiolucent lesions, low to intermediate attenuation, usually shows contrast enhancement (usually heterogeneous); locally invasive associated with bone erosion/destruction; usually involves the dorsal portion of the vertebral body with extension toward the spinal canal. Also occurs in sacrum. |
Rare, slow-growing tumors (~3% of bone tumors); usually occur in adults 30 to 70 y old; M > F (2:1); sacrum (50%) > skull base (35%) > vertebrae (15%). |
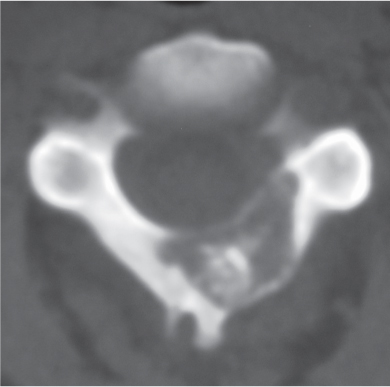
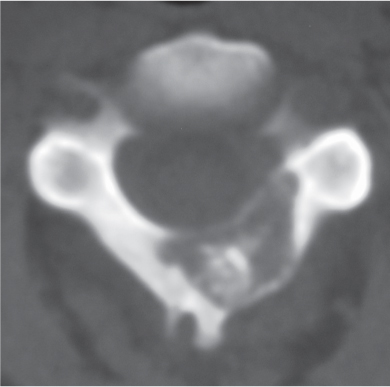
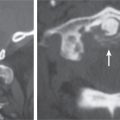
Chondrosarcoma
Fig. 10.13a–c |
Lobulated radiolucent lesions, low to intermediate attenuation, with or without matrix mineralization; may show contrast enhancement (usually heterogeneous); locally invasive associated with bone erosion/destruction, encasement of vessels and nerves; can involve any portion of the vertebra. |
Rare, slow-growing malignant cartilaginous tumors (~16% of bone tumors), usually occur in adults (peak in fifth to sixth decades), M > F; sporadic (75%), malignant degeneration/transformation of other cartilaginous lesion, enchondroma, osteochondroma, etc. (25%). |
Osteogenic sarcoma
Fig. 10.14a, b |
Destructive malignant lesions, low to high attenuation, usually with matrix mineralization/ossification within lesion or within extraosseous tumor extension; can show contrast enhancement (usually heterogeneous). Cortical bone destruction and epidural extension of tumor can compress the spinal canal and spinal cord. |
Malignant bone lesions rarely occur as primary tumor involving the vertebral column; locally invasive, high metastatic potential. Occurs in children as primary tumors and adults associated with Paget disease, irradiated bone, chronic osteomyelitis, osteoblastoma, giant cell tumor, and fibrous dysplasia. |
Ewing sarcoma |
Destructive malignant lesions involving the vertebral column, radiolucent with low to intermediate attenuation; typically lack matrix mineralization; can show contrast enhancement (usually heterogeneous). Cortical bone destruction and epidural extension of tumor can compress the spinal canal and spinal cord. |
Usually occurs between the ages of 5 and 30, M > F; rarely occurs as primary tumor involving the spinal column; locally invasive, high metastatic potential. |
Malignant fibrous histiocytoma (MFH) |
Tumors are often associated with zones of cortical destruction and extraosseous soft tissue masses. Tumors have low to intermediate attenuation, can show contrast enhancement. Cortical bone destruction and epidural extension of tumor can compress the spinal canal and spinal cord. |
Malignant tumors involving soft tissue and rarely bone that are presumed to derive from undifferentiated mesenchymal cells. The World Health Organization (WHO) now uses the term undifferentiated pleomorphic sarcoma for pleomorphic MFH. |
Hemangioendothelioma |
Lesions usually have sharp margins that may be slightly lobulated and often have low to intermediate attenuation; can be intraosseous radiolucent lesions or extradural soft tissue lesions. Can be multifocal. Extraosseous extension of tumor through zones of cortical destruction can be seen. Lesions can show contrast enhancement. |
Vasoformative/endothelial low-grade malignant neoplasms that are locally aggressive and rarely metastasize compared with high-grade angiosarcoma. |
Hemangiopericytoma |
Tumors often have well-defined margins; intraosseous lesions can be radiolucent with or without lobulated margins; extraosseous lesions can have low to intermediate attenuation. Lesions may contain slightly prominent vessels centrally or peripherally, with or without hemorrhagic zones. Can show contrast enhancement. |
Rare malignant tumors of pericytic origin that occur in soft tissues and less frequently in bone. |
Neoplasms (benign) |
Enchondroma |
Lobulated radiolucent lesions, low to intermediate attenuation, with or without matrix mineralization; can show contrast enhancement (usually heterogeneous). Locally invasive associated with bone erosion/destruction; usually involves posterior elements. |
Rare, slow-growing tumors (~12% of bone tumors); usually occur in children and young adults (10–30 y), M > F. |
Chondroblastoma |
Tumors are typically radiolucent with lobular margins and typically have low to intermediate attenuation. Up to 50% have chondroid matrix mineralization. May show contrast enhancement. Cortical destruction is uncommon. Bone expansion secondary to the lesion can result in spinal cord compression. |
Benign cartilaginous tumors with chondroblast-like cells and areas of chondroid matrix formation that rarely occur in the spine. Spinal tumors most often involve the thoracic vertebrae and usually involve both the body and pedicles. |
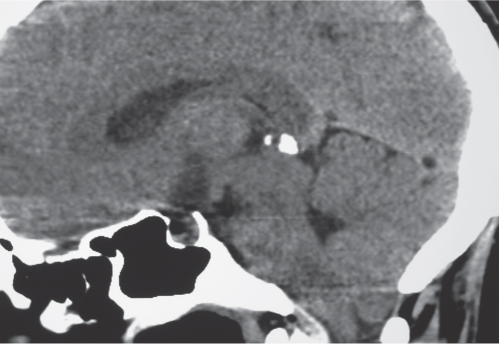
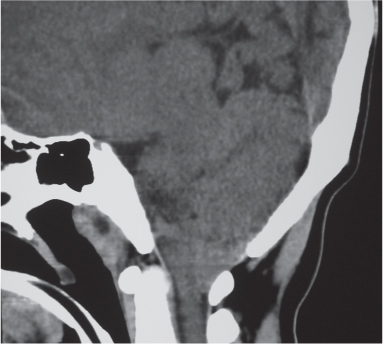
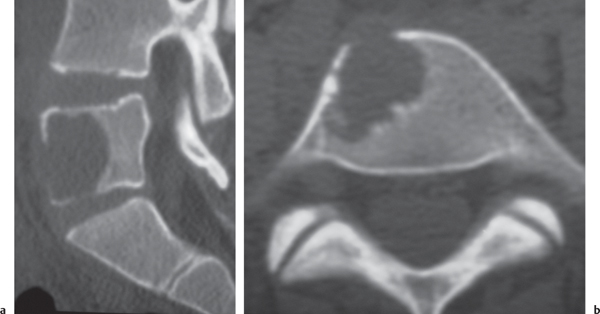
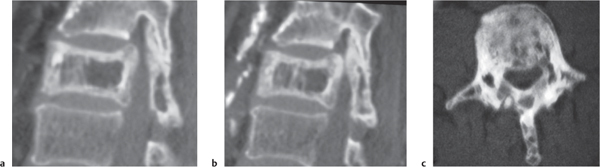
Osteoid osteoma
Fig. 10.15 |
Intraosseous circumscribed radiolucent lesion often < 1.5 cm in diameter located in posterior elements, central zone with low to intermediate attenuation that can show contrast enhancement, surrounded by a peripheral zone of high attenuation (reactive bone sclerosis). |
Benign osseous lesion containing a nidus of vascularized osteoid trabeculae surrounded by osteoblastic sclerosis; 14% of osteoid osteomas are located in the spine; usually occurs between the ages of 5 and 25 y, M > F. Focal pain and tenderness associated with lesion are often worse at night, relieved with aspirin. |
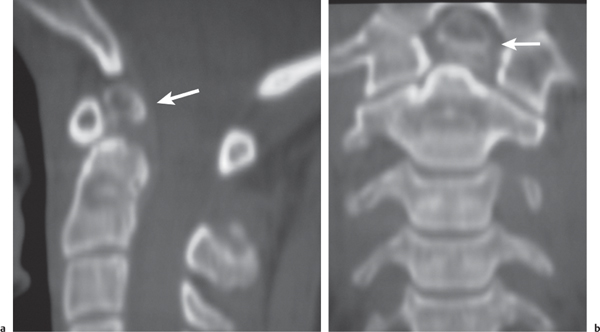
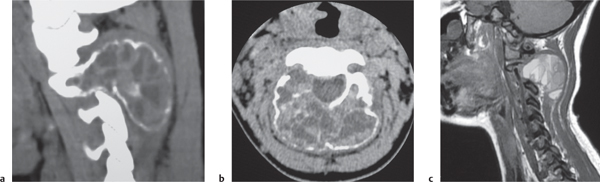
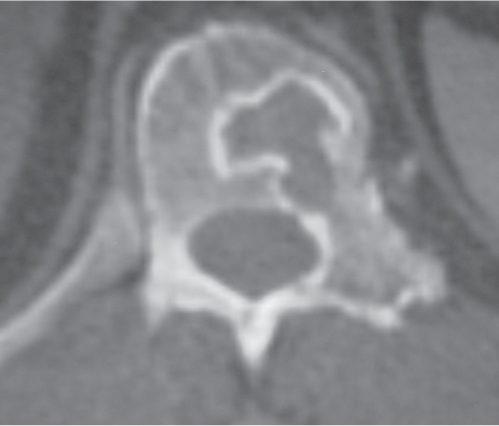
Osteoblastoma
Fig. 10.16 |
Expansile radiolucent vertebral lesion often < 1.5 cm in diameter located in posterior elements (90%) with or without extension into vertebral body (30%), with or without epidural extension (40%), low to intermediate attenuation, often surrounded by a zone of bone sclerosis; can show contrast enhancement, with or without spinal cord/spinal canal compression. |
Rare benign bone neoplasm (2% of bone tumors) usually occurs between age 6 and 30 y. One third of osteoblastomas involve the spine. |
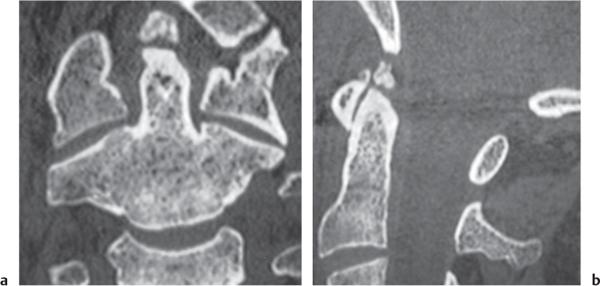
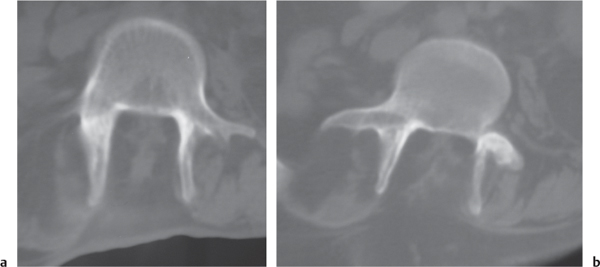
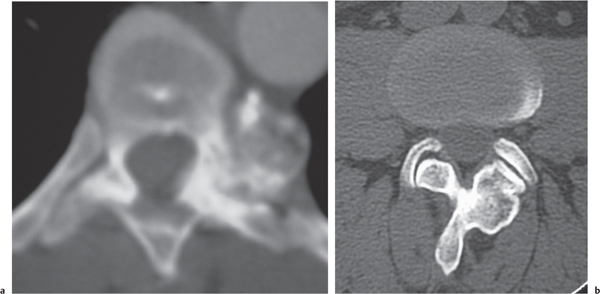
Giant cell tumor
Fig. 10.17a, b |
Circumscribed radiolucent vertebral lesion with low to intermediate attenuation; can show contrast enhancement. Location: vertebral body > vertebral body and vertebral arch > vertebral arch alone. With or without spinal cord/spinal canal compression, with or without pathologic fracture. |
Locally aggressive lesions that rarely metasta-size. Account for 5% of primary bone tumors. Usually involve lone bones; only 4% involve vertebrae. Occur in adolescents and adults (age 20–40 y). |
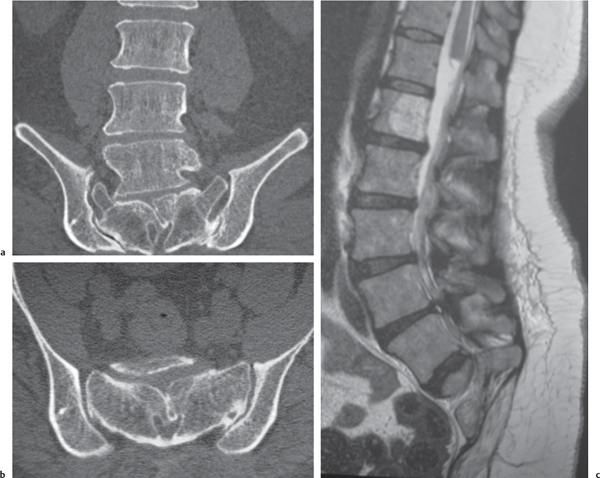
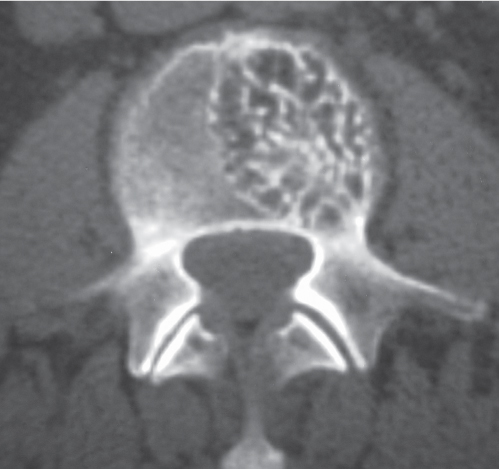
Aneurysmal bone cyst
Fig. 10.18a–c |
Circumscribed vertebral lesion usually involving the posterior elements with or without involvement of the vertebral body; variable low, intermediate, high, and/or mixed attenuation, with or without surrounding thin shell of bone, with or without lobulations, with or without one or multiple fluid/fluid levels, with or without pathologic fracture. |
Expansile blood/debris-filled lesions that may be primary or occur secondary to other bone lesions, such as giant cell tumor, fibrous dysplasia, and chondroblastoma. Most occur in patients younger than 30 y. Locations: lumbar > cervical > thoracic. Clinical findings can include neurologic deficits and pain. |
Osteochondroma
Fig. 10.19a, b |
Circumscribed sessile or protuberant osseous lesion typically arising from posterior elements of vertebrae, central zone contiguous with medullary space of bone, with or without cartilaginous cap. Increased malignant potential when cartilaginous cap is > 2 cm thick. |
Benign cartilaginous tumors arising from defect at periphery of growth plate during bone formation with resultant bone outgrowth covered by a cartilaginous cap.
Usually benign unless associated with pain and increasing size of cartilaginous cap. Can occur as multiple lesions (hereditary exostoses) with increased malignant potential. |
Hemangioma
Fig. 10.20 |
Circumscribed or diffuse vertebral lesion usually radiolucent without destruction of bone trabeculae, located in the vertebral body with or without extension into pedicle or isolated within pedicle; typically low to intermediate attenuation with thickened vertical trabeculae; can show contrast enhancement; multiple in 30%. Location: thoracic (60%) > lumbar (30%) > cervical (10%). |
Most common benign lesions involving vertebral column, F > M, composed of endotheliallined capillary and cavernous spaces within marrow associated with thickened vertical trabeculae and decreased secondary trabeculae; seen in 11% of autopsies. Usually asymptomatic; rarely cause bone expansion and epidural extension resulting in neural compression (usually in thoracic region); increased potential for fracture with epidural hematoma. |
Other tumorlike lesions |
Paget disease
Fig. 10.21a–c |
Expansile sclerotic/lytic process involving a single or multiple vertebrae with mixed intermediate to high attenuation. Irregular/indistinct borders between marrow cortical bone; can also result in diffuse sclerosis, “ivory” vertebral pattern. |
Chronic disease with disordered bone resorption and woven bone formation. Usually seen in older adults; polyostotic in 66%; can result in narrowing of neuroforamina and spinal canal. |
Fibrous dysplasia
Fig. 10.22 |
Expansile process involving one or more vertebrae with mixed intermediate and high attenuation, often a “ground glass” appearance. |
Benign medullary fibro-osseous lesion of bone usually seen in adolescents and young adults; can result in narrowing of the spinal canal and neuroforamina; mono- and polyostotic forms (with or without endocrine abnormalities such as with McCune-Albright syndrome/precocious puberty). |
Arachnoid cyst |
Well-circumscribed, extra-axial lesions with low attenuation similar to CSF. No contrast enhancement. Usually cause mass effect on the adjacent spinal cord. Chronic erosive changes can be seen at the vertebrae adjacent to the cyst. |
Nonneoplastic acquired, developmental, or congenital extra-axial cysts filled with CSF. Cysts can be small or large, asymptomatic or symptomatic. |
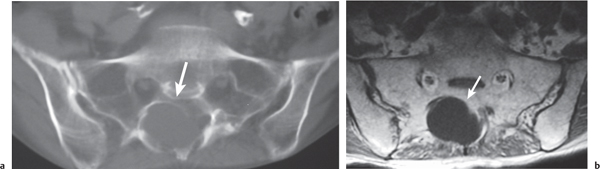
Tarlov cyst (perineural cyst)
Fig. 10.23a, b |
Well-circumscribed cysts with CSF attenuation involving nerve root sleeves associated with chronic erosive changes involving adjacent bony structures. Sacral (with or without widening of sacral foramina) > lumbar nerve root sleeves. Usually range from 15 to 20 mm in diameter but can be larger. |
Typically represent incidental asymptomatic anatomical variants associated with prior dural injury. |
Dermoid |
Well-circumscribed, spheroid or multilobulated, intradural extramedullary or intramedullary lesions that can contain zones with low, intermediate, and/or high attenuation and calcifications; usually show no contrast enhancement, with or without fluid–fluid or fluid–debris levels. Lumbar region most common location in spine. Can cause chemical meningitis if dermoid cyst ruptures into the subarachnoid space. Commonly located at or near midline. |
Nonneoplastic congenital or acquired ectodermal inclusion cystic lesions filled with lipid material, cholesterol, desquamated cells, and keratinaceous debris; usually mild mass effect on adjacent spinal cord or nerve roots. Adults: M slightly > F; with or without related clinical symptoms. |
Epidermoid |
Well-circumscribed, spheroid or multilobulated, intradural extramedullary lesion with low to intermediate attenuation; typically shows no contrast enhancement. |
Nonneoplastic extramedullary epithelial- inclusion lesions filled with desquamated cells and keratinaceous debris; usually mild mass effect on adjacent spinal cord and/or nerve roots. May be congenital (with or without associated with dorsal dermal sinus, spina bifida, hemivertebrae) or acquired (late complication of lumbar puncture). |
Neurenteric (endodermal) cyst |
Well-circumscribed, spheroid, intradural extramedullary lesions with low to intermediate attenuation; usually show no contrast enhancement. Lesions may extend into the spinal cord in 10%. |
Developmental failure of separation of noto-chord and foregut resulting in sinus tract or cysts between ventrally located endoderm and dorsally located ectoderm. Distal long tract symptoms and progressive spinal cord compression. |
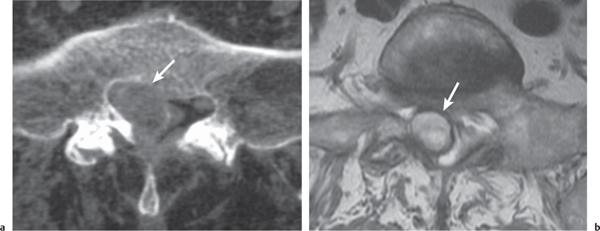
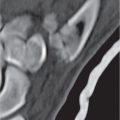
Synovial cyst
Fig. 10.24a, b |
Circumscribed lesion located adjacent to the facet joint. A thin rim of intermediate attenuation surrounds a central zone that may have low to intermediate attenuation. No contrast enhancement is usually seen, but a thin rim of peripheral enhancement may be observed. |
Represents protrusion of synovium with fluid from degenerated facet joint into the spinal canal medially or dorsally into the posterior paraspinal soft tissues. Variable CT attenuation and MRI signal is related to the contents, which may include serous or mucinous fluid, blood, hemosiderin, and/or gas. |
Bone island |
Usually appears as a circumscribed radiodense ovoid or spheroid focus in medullary bone that may or may not contact the endosteal surface of cortical bone. |
Bone islands (enostoses) are nonneoplastic intramedullary zones of mature compact bone composed of lamellar bone that are considered to be developmental anomalies resulting from localized failure of bone resorption during skeletal maturation. |
Trauma |
Fracture
Fig. 10.25
Fig. 10.26 |
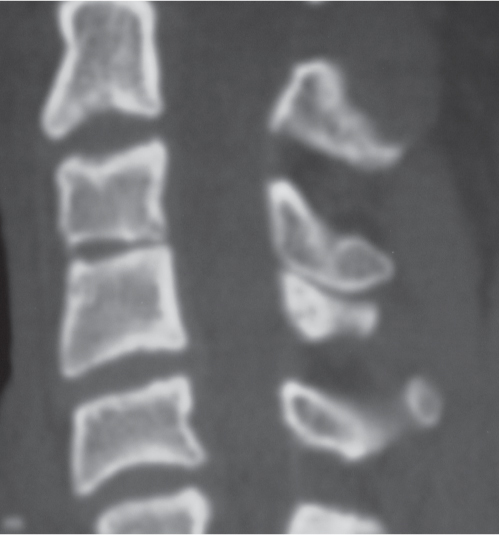
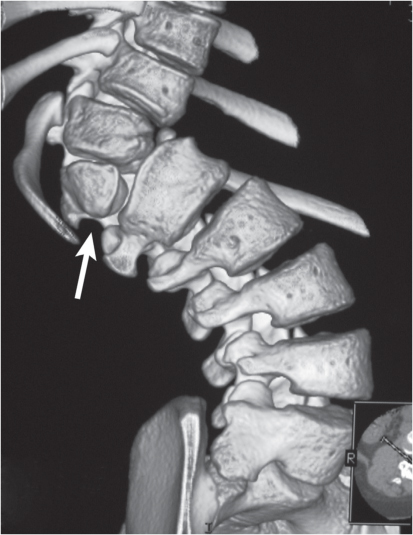
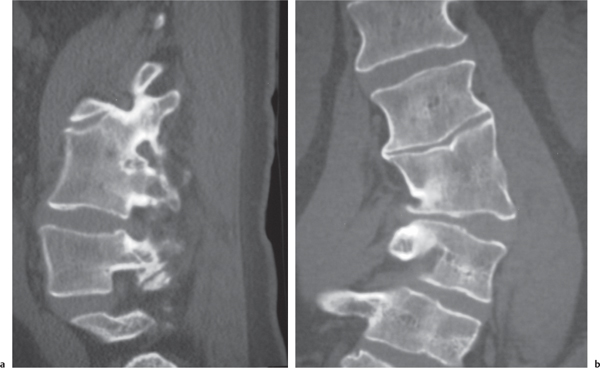
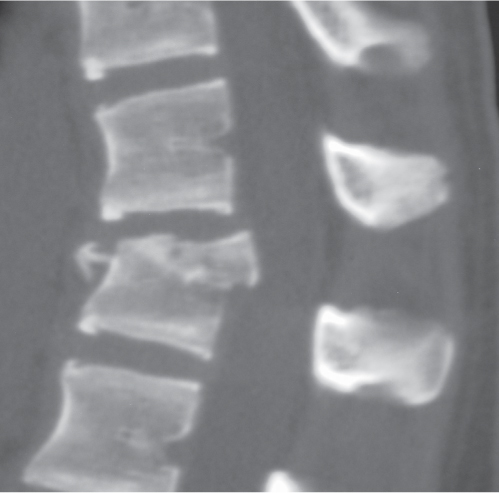
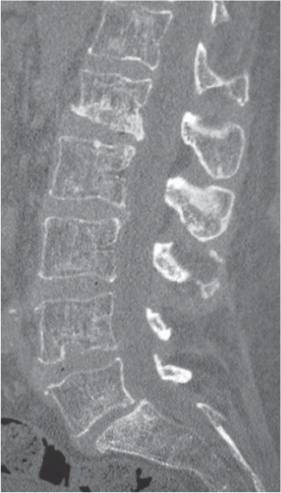
Traumatic and osteopenic vertebral fracture: Acute/subacute fractures have sharply angulated cortical margins, no destructive changes at cortical margins of fractured end plates, with or without convex outward angulated configuration of compressed vertebral bodies, with or without spinal cord and/or spinal canal compression related to fracture deformity, with or without retropulsed bone fragments into spinal canal, with or without subluxation, with or without kyphosis, with or without epidural hematoma.
Malignancy-related vertebral fracture: Fractures related to radiolucent and/or sclerotic lesions, with or without destructive changes at cortical margins of vertebrae, with or without convex outward-bowed configuration of compressed vertebral bodies, with or without paravertebral mass lesions, with or without spheroid or poorly defined lesions in other noncompressed vertebral bodies. |
Vertebral fractures can result from trauma, primary bone tumors/lesions, metastatic disease, bone infarcts (steroids, chemotherapy, and radiation treatment), osteoporosis, osteomalacia, metabolic (calcium/phosphate) disorders, vitamin deficiencies, Paget disease, and genetic disorders (osteogenesis imperfecta, etc.). |
Inflammation/infection |
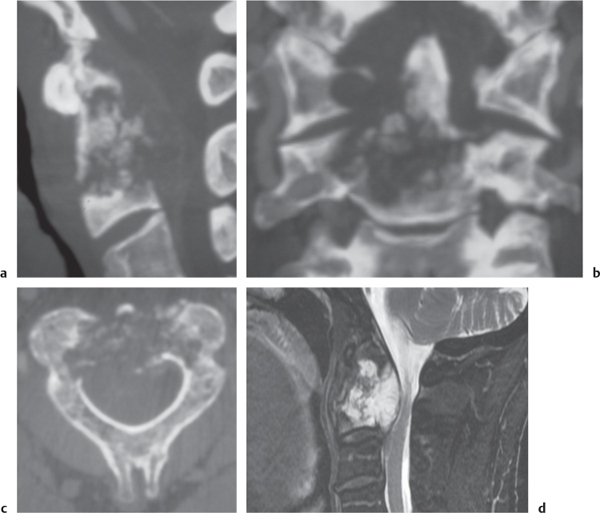
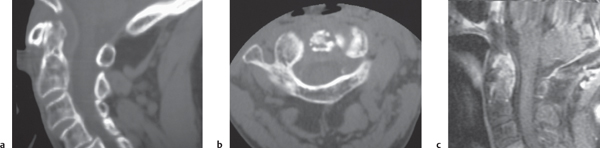
Rheumatoid arthritis
Fig. 10.27a–c |
Erosions of vertebral end plates, spinous processes, and uncovertebral and apophyseal joints. Irregular enlarged enhancing synovium (pannus: low to intermediate attenuation) at atlantodens articulation results in erosions of dens and transverse ligament, with or without destruction of transverse ligament with C1 on C2 subluxation and neural compromise; with or without basilar impression. |
Most common type of inflammatory arthropathy that results in synovitis, causing destructive/erosive changes of cartilage, ligaments, and bone. Cervical spine involvement in two thirds of patients, juvenile and adult types. |
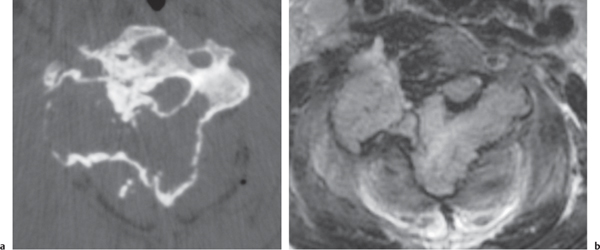
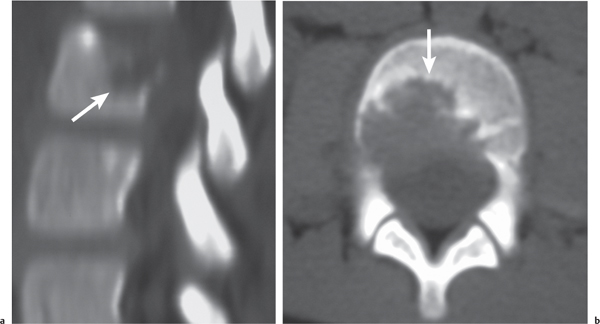
Eosinophilic granuloma
Fig. 10.28a, b |
Single or multiple circumscribed radiolucent lesions in the vertebral body marrow associated with focal bony destruction/erosion with extension into the adjacent soft tissues. Lesions usually have low to intermediate attenuation and involve the vertebral body and not the posterior elements; can show contrast enhancement, with or without enhancement of the adjacent dura. Progression of the lesion can lead to vertebra plana (a collapsed flattened vertebral body), with minimal or no kyphosis and relatively normalsized adjacent disks. |
Single lesion: Commonly seen in male patients younger than 20 y; proliferation of histiocytes in medullary cavity with localized destruction of bone with extension into adjacent soft tissues.
Multiple lesions: Associated with syndromes such as Letterer–Siwe disease (lymphadenopathy, hepatosplenomegaly), children younger than 2 y; Hand–Schüller–Christian disease (lymphadenopathy, exophthalmos, and diabetes insipidus), children between 5 and 10 y. |
Hematopoietic |
Amyloidoma |
Amyloid lesions in bone can occur as zones of osteopenia, permeative radiolucent destruction or uni- or multifocal radiolucency. Lesions can have low to intermediate attenuation and can show contrast enhancement. |
Uncommon disease in which various tissues (including bone, muscle, tendons, tendon sheaths, ligaments, and synovium) are infiltrated with extracellular eosinophilic material composed of insoluble proteins with beta-pleated sheet configurations (amyloid protein). Amyloidomas are single sites of involvement. Amyloidosis can be a primary disorder associated with an immunologic dyscrasia or secondary to a chronic inflammatory disease. |
Bone infarcts |
Focal ringlike lesion or poorly defined zone with increased attenuation in medullary bone; usually no contrast enhancement, with or without associated fracture. |
Bone infarcts can occur after radiation treatment, surgery, corticosterioid medications, chemotherapy, or trauma. |
Congenital |
Myelomeningocele/myelocele |
Imaging is usually performed after surgical repair of myeloceles or myelomeningoceles. Posterior protrusion of spinal contents is seen with unfolded neural tube (neural placode) through defects in the bony dorsal elements of the involved vertebrae or sacral elements. The neural placode is usually located at the lower lumbar-sacral region with resultant tethering of the spinal cord. If the neural placode is flush with the adjacent skin surface, the anomaly is labeled a myelocele. If the neural placode extends above the adjacent skin surface, the anomaly is labeled a myelomeningocele, with or without syringohydromyelia. |
Failure of developmental closure of the caudal neural tube results in an unfolded neural tube (neural placode) exposed to the dorsal surface in the midline without overlying skin. Other features associated with myelomeningoceles and myeloceles are dorsal bony dysraphism, deficient dura posteriorly at the site of the neural placode, and Chiari II malformations. By definition, the spinal cords are tethered. Usually repaired surgically soon after birth. |
Meningoceles |
Protrusion of CSF and meninges through a dorsal vertebral defect either from surgical laminectomies or congenital anomaly. Sacral meningoceles can alternatively extend anteriorly through a defect in the sacrum. |
Acquired meningoceles are more common than meningoceles resulting from congenital dorsal bony dysraphism. Anterior sacral meningoceles can result from trauma or be associated with mesenchymal dysplasias (NF1, Marfan syndrome, and syndrome of caudal regression). |
Lipomyelomeningocele |
Unfolded caudal neural tube (neural placode) covered by a lipoma that is often contiguous with the dorsal subcutaneous fat through defects (spina bifida) involving the bony dorsal vertebral elements. The neural placode is usually located at the lower lumbar-sacral region with resultant tethering of the spinal cord, with or without syringohydromyelia. |
Failure of developmental closure of the caudal neural tube results in an unfolded neural tube (neural placode) covered by a lipoma that is contiguous with the subcutaneous fat. The overlying skin is intact, although the lipoma usually protrudes dorsally. The nerve roots arise from the placode. Features associated with lipomyelomeningoceles and lipomyeloceles include tethered spinal cords, dorsal bony dysraphism, and deficient dura posteriorly at the site of the neural placode. Not associated with Chiari II malformations. Diagnosis often in children, occasionally in adults. |