Abstract
The goal of MRI is to establish the size and extent of the known index malignancy, to identify additional sites of malignancy and to improve surgical treatment planning. It has been well documented that multifocal and diffuse (multicentric) breast cancer is an independent negative prognostic factor, affecting therapeutic decision-making. MRI allows precise identification not only of the index lesion, be it invasive or noninvasive, but also the presence and extent of additional previously undetected cancer. Identification of multifocal, multicentric, diffuse and contralateral tumors not only helps to guide treatment planning but also provides an independent negative prognostic indicator. In this chapter we discuss the literature that relates to the use of breast MRI for assessment of disease in the preoperative setting, and the increasing evidence of the efficacy of preoperative MRI in decreasing re-operation rates.
Many practices now rely on MRI for monitoring of assessment response during neoadjuvant systemic chemotherapy (NACT; chemotherapy prior to surgery for invasive cancer) and is widely used to provide improved surgical outcomes, recurrence free survival and overall survival in certain subtypes of breast cancer, with the additional potential benefit that reduced tumor volume during treatment may allow for more conservative surgery. MRI also allows superior assessment of tumor size, volume and surface area measurements reflecting a more accurate characterization of overall tumor burden especially during NACT monitoring of tumor response. Diagnostic MRI is also used for management of residual disease following cancer excision and evaluation of suspected recurrent disease following breast conserving surgery.
10 Diagnostic MRI: Breast Cancer Applications
10.1 Introduction
The increased numbers of small cancers detected at mammography screening allow breast-conserving surgery (BCS) for many women, underscoring the importance of accurate assessment of tumor extent. Traditional breast cancer treatment is determined by two major factors: tumor histology, assessed by classifications based on grade and morphology, and the TNM staging method, based on cancer size, nodal status, and presence or absence of distant metastases. Breast cancer therapy relies primarily on surgical treatment when cancers are nonpalpable and detected at screening. Complete imaging evaluation for each individual cancer patient should guide the surgical approach. In the pre–magnetic resonance imaging (MRI) era, surgical and oncologic treatment planning methods were judged primarily by clinical examination, mammography, and ultrasound imaging. Precise assessment of tumor size at mammography was often difficult to achieve, particularly for the patient with dense breast tissue, resulting in the large variation in postsurgical positive margins reported in the literature (5–70%). 1 Breast MRI provides superior sensitivity and accuracy in determining the tumor burden in patients with newly diagnosed breast cancer. The goal of MRI is to establish the size and extent of the known index malignancy, to identify additional sites of malignancy, and to improve surgical treatment planning. MRI allows precise identification not only of the index lesion, be it invasive or noninvasive, but also of the presence and extent of additional previously undetected cancer. It has been well documented that multifocal and diffuse (multicentric) breast cancer is an independent negative prognostic factor, affecting therapeutic decision-making. 2 , 3 MRI allows identification of 15% (12–27%) additional occult malignancies in the ipsilateral breast and 4% (3–6%) in the contralateral breast. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 Accurate MRI depiction of disease extent may serve patients well by selecting those patients with a large tumor burden at initial diagnosis, thus avoiding immediate lumpectomy and allowing change in therapy to mastectomy or neoadjuvant chemotherapy. MRI also plays an important role in the assessment of tumor burden when margins are positive at lumpectomy, when recurrent tumor is suspected, and when monitoring tumor response for the patient undergoing neoadjuvant chemotherapy.
10.2 Imaging of the Patient with Known Cancer
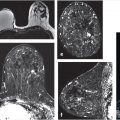
Breast cancer is known to be a heterogeneous disease with characteristic molecular and genetic subtypes, and although the traditional histologic classification of breast cancer offers limited prognostic value, molecular characterization, cellular markers, and imaging phenotypes of breast tumors, especially MRI, have changed the treatment landscape. Most breast cancers are multifocal at histology, with MRI identifying a substantial number of additional lesions, both invasive and noninvasive, in the ipsilateral and contralateral breast. A report on 500 consecutive breast cancer cases, retrospectively analyzed and documented by large-format histologic sectioning, determined the distribution of both in situ and invasive components of breast tumors. The large-section histology method has proven to be the most accurate way of achieving precise estimation of malignancy extent. The distribution of cancer found in this study was follows: unifocal 34%, multifocal 36%, diffuse 28%, and mixed 2%. It is not surprising therefore that the exquisite sensitivity of MRI allows detection of additional disease not identified by other imaging methods (Fig. 10‑1, Fig. 10‑2). 13


Preoperative MRI can assess disease extent more accurately than other imaging techniques, and over time may also provide improved prognostication and prediction. Quantitative analyses of image-based phenotypes have opened the door to provision of MR image–based predictive and prognostic biomarkers. A biomarker is defined as a characteristic that is objectively measured and evaluated as an indicator of normal biologic or pathogenic processes, or a pharmacological response to a therapeutic intervention that could aid in therapeutic decision-making. MRI tumor characteristics have been shown to correlate with both histologic and molecular subgroups, as shown in Chapter 8. Although gene expression profiling is increasingly used for treatment planning, immunohistochemical (IHC) markers are commonly used as substitute measures of tumor biology. These markers include the presence of estrogen and progesterone receptors (ER, PR), overexpression of the oncogene HER-2/neu, and proliferation rate, as measured by Ki-67. Imaging biomarkers need confirmation of accuracy and efficacy to be appropriately validated as surrogate end points. Their measurements should be accurate and reproducible and closely connected to the target lesion or treatment effect. Quantitative radiomics, a discipline that has developed quantitative image-based techniques with clinical applications such as cancer risk assessment and risk recurrence, are discussed further in Chapter 13.
Diagnostic accuracy is improved when preoperative MRI is performed, prompting changes in patient management. However, both overestimation and underestimation of disease can occur and confirmative biopsy, of suspicious lesions found only at MRI, is necessary before changes in therapeutic management are put into effect. The decision to change the surgical approach from lumpectomy to mastectomy based on MRI findings, for example, should only be made after biopsy proof of additional cancer (Fig. 10‑3; Fig. 10‑4). Cancer patients will benefit if treatment planning is made after careful review of all clinical, imaging, and histologic findings in a multidisciplinary setting. Individualized therapeutic regimens can aid in determining optimal patient outcome.


10.2.1 Tumor Measurements
Accurate tumor measurements are necessary for surgical and oncologic treatment planning, not only at initial diagnosis, but also when monitoring chemotherapeutic treatment. It is evident that tumor multifocality identified at MRI cannot be accurately assessed in most cases by a single maximum diameter measurement of the index cancer and satellites. 14 Complete assessment of the tumor burden requires measurement of not only the size of the individual tumor component(s) but also the full volume of the extent of disease, both invasive and in situ. Multifocal disease may present as in situ cancer only, multiple invasive cancers with intervening normal tissue, or multiple invasive cancers associated with an in situ component, necessitating carefully imaging and histologic correlation. It is logical therefore that breast cancer patients might benefit from the accurate MRI identification of multifocal and diffuse disease that would otherwise go undetected. It has been shown in many studies that the use of multimodal imaging, and the additional use of percutaneous biopsy techniques, can provide precise maps of the extent and localization of breast disease. Computer-generated volume and surface area calculations reflect a more accurate estimate of tumor burden and can be automatically generated using newer advanced analytic software.
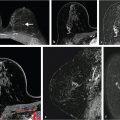
In rare circumstances, a patient may present with enlarged axillary lymph nodes, biopsy indicating metastatic disease likely of breast origin; however, clinical breast examination, mammography, and ultrasound studies are normal. MRI is usually able to identify the primary lesion if it indeed originates in the ipsilateral breast (Fig. 10‑5; Fig. 10‑6).


MRI frequently detects ductal carcinoma in situ (DCIS) and associated invasive components not seen on mammography. Holland et al reported that mammographically occult DCIS was present histologically in 16% of higher-grade DCIS lesions larger than 20 mm, with necrosis, whereas occult disease was found in 47% cases with predominantly micropapillary-cribriform growth patterns. 15 Women undergoing surgical excision of DCIS diagnosed only at mammography may experience an inadequate surgical resection resulting in positive margins and the need for re-excision surgery. Moreover, axillary nodal metastases may be found in 5 to 15% of patients with DCIS diagnosed at mammography; sentinel node (SLN) biopsy is justified in women with DCIS who have a high risk of invasive carcinoma, such as those with large tumors, a mass, or high-grade lesions. 16 MRI is able to detect the extent of DCIS and identify foci of invasion not present on mammography and ultrasound (Fig. 10‑7; Fig. 10‑8).


10.3 Breast-Conserving Therapy (BCS)
BCS is used as an alternative to mastectomy for patients with diagnosis of early-stage breast cancer and is the most commonly performed breast surgical procedure. Partial mastectomy, or lumpectomy, consists of surgical removal of the tumor and the immediate surrounding normal breast tissue. Lumpectomy followed by radiation therapy (RT) has been shown in randomized clinical trials to be as effective as mastectomy when disease-free and overall survival rates are calculated. 17 , 18 , 19 , 20 , 21 The goal for successful BCS requires that the entire malignant lesion be removed with margins negative for tumor; therefore, knowledge of the true extent of the cancer is an important requisite for successful surgery. The literature is varied, however, as to whether MR identification of additional ipsilateral or contralateral occult malignancy is beneficial, given current therapeutic regimens.
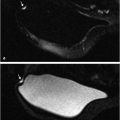
Factors influencing treatment options among patients considered suitable for BCS include tumor size, location, nipple involvement, and presence or absence of tumor multifocality or multicentricity/diffuse disease. Additional considerations include tumor size as related to breast volume, cosmetic options, and patient preference. Identification of additional cancers can lead to a more extensive lumpectomy than originally planned, or even a mastectomy. In clinical practice, most patients have received a percutaneous biopsy prior to the MR examination and a postbiopsy seroma may be visible (Fig. 10‑9). MRI may detect an occult invasive component in a region of previously diagnosed DCIS, for example, or demonstrate axillary and internal mammary lymphadenopathy, all of which can impact surgical management. Preoperative MRI planning is especially useful for detection of mammographically occult DCIS. In some cases, DCIS may be found to be much more extensive on MR than on mammography and findings may even extend to the large subaerolar ducts. Careful discussion of preoperative MR findings between the radiologist and the surgeon is necessary for favorable outcome. Two recent papers investigated the efficacy of preoperative breast MR imaging, MR-guided surgery and surgical outcome, showing improved depiction of disease extent and resulting change in therapeutic management (Fig. 10‑10; Fig. 10‑11). 22 23



MRI is also very useful for identification of the associated features of malignancy, identified in Table 10‑1.
Nipple retraction | Axillary adenopathy |
Nipple invasion | Pectoralis muscle invasion |
Skin retraction | Chest wall invasion |
Skin thickening | Architectural distortion |
Skin invasion: direct invasion, inflammatory cancer | |
Source: Morris EA, Comstock CE, Lee CH, et al. ACR BI-RADS® Magnetic Resonance Imaging. In: ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA, American College of Radiology; 2013. | |
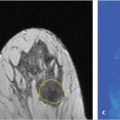
Important benefits of preoperative MRI include identification of tumor invasion extending deep to fascia and determination of the relationship of tumor to fascia and its extension into the pectoralis major, serratus anterior, and/or intercostal muscles. MRI can detect muscle involvement, which impacts tumor staging, surgical planning, and the overall therapeutic approach—an extremely difficult, if not impossible, task to diagnose on clinical examination, mammography, or ultrasound. 22 , 23 Careful MR assessment is needed for diagnosis of pectoral muscle invasion. Enhancement that approaches or violates the prepectoral fat plane is not sufficient. Enhancement within the muscle itself is necessary for detection of invasion, superficial muscle enhancement allowing partial muscle excision, whereas radical mastectomy may be needed if the full thickness of the muscle is involved with tumor (Fig. 10‑12; Fig. 10‑13).


Nipple and subareolar duct involvement can be seen either by direct involvement from an anterior invasive cancer or by linear enhancing extension of a DCIS lesion. 24 , 25 Surgeons will usually resect the nipple–areola complex if tumor is documented. MRI can identify DCIS lesions that exhibit linear enhancement extending into the subareolar region close to the nipple, which are occult at mammography (Fig. 10‑14). Skin enhancement suggests skin involvement with malignancy. Skin edema and skin thickening may be due to lymphatic obstruction with or without malignant involvement. Direct skin infiltration from an underlying malignant lesion is generally evident clinically and is well seen on MRI (Fig. 10‑15; Fig. 10‑16). 26 , 27



10.4 Management of Additional Disease
Identification of additional invasive cancer or DCIS at preoperative MRI has changed surgical management, by allowing a more accurate assessment of disease extent. When additional suspicious occult foci or masses are identified, image-guided percutaneous biopsy of the MR findings is usually required if the presence of cancer will alter surgical management. Identification of unsuspected invasive lesion(s) associated with a DCIS diagnosis will often change surgical management of the axilla. BI-RADS 3 assessments in this setting are generally not warranted as a specific diagnosis is needed.
Mapping of MRI-only visible DCIS in the preoperative setting often requires MR-guided biopsy (or biopsies) and clip placement(s) to document lesion extent. Multiple-wire placement is often needed to bracket imaging findings, resulting in complex surgical excisions. Successful outcome requires close collaboration between the surgeon, radiologist, and pathologist on the day of the surgical procedure. Despite improved DCIS mapping with MRI, some reports suggest that this capability does not always result in a decrease in the number of patients with positive margins at surgical excision. 15 Translation of imaging data from the magnet to the surgical environment is challenging. One explanation might relate to the position of the patient, who is supine in the operating room and prone in the magnet. Correlation of lesion(s) location using prone MR images for guidance may prove to be difficult for the surgeon, particularly when attempting to resect multifocal disease. Contributing to this problem is the surgical approach, which may be oriented orthogonally to the length of the target DCIS lesion, thus increasing the difficulty of complete resection, even when the lesions are appropriately bracketed. Examples of the difference between the location of breast tissue in the prone and supine positions are shown (Fig. 10‑17; Fig. 10‑18).


10.5 Reoperation Rates
A review of the literature indicates that the frequency of tumor in second surgical excision specimens ranges from 32 to 63%, with microscopic residual disease being found in about 50% of patients overall. 28 , 29 Findings of residual tumor have been shown to be associated with an increasing risk of recurrent cancer in the ipsilateral breast, for both invasive and noninvasive cancer, with some investigators showing increased local recurrence rates. 30 , 31 Other reports have indicated no increase in recurrence rates. 32 , 33 , 34 A retrospective observational trial conducted in four large institutions in the United States between 2003 and 2008 evaluated 2,206 women with 2,220 invasive breast cancers undergoing partial mastectomy. 1 The aim was to assess hospital- and surgeon-specific variation in reoperation rates following initial surgery. Overall, 22.9% patients underwent additional surgery on the affected breast. Among these patients, 89.2% underwent a single operative procedure, 9.4% underwent two re-excisions, and 4% underwent three re-excisions. Subsequent mastectomy was required in 8.4%. The authors concluded that substantial surgeon and institutional variation existed; however, re-excision rates were somewhat lower than previously reported rates of 36 to 50%. 35 , 36 Morrow et al reported a 37.9% re-excision rate following initial BCS in a similarly large series of 1,459 patients in 2009, with 26.0% patients undergoing local repeat surgery and 11.9% ultimately undergoing mastectomy. 35
Another study published in 2016 showed that nearly one in four women who underwent BCS in New York State between 2011 and 2013 required a reoperation within 90 days. 37 The reoperation rate averaged 30%, decreasing from a 38.5% rate during 2003 to 2005, to a 23.1% rate during 2011 to 2013. It is important to note that surgeons with higher volume of procedures were independently associated with lower reoperation rates, whereas procedures performed by low-volume surgeons were associated with a 50% higher risk for reoperation, the difference persisting in multivariable analyses.
A modified surgical technique known as “cavity shaving” involves re-excision of the entire surgical cavity by removing additional tissue from each face of the cavity at time of initial cancer resection. This surgical method has proved successful as reported in a recent randomized trial, by significantly reducing the rate of positive margins from 34 to 19% and the rate of re-excision from 21 to 10%, in the “shaved” versus the standard surgical approach. 38
10.5.1 Role of MRI for Reducing Rates of Re-excision
Can preoperative breast MRI help reduce reoperation rates by providing careful mapping of disease extent? Questions remain as to whether the improved sensitivity of preoperative breast MRI improves patient outcomes and the use of MRI for staging of newly diagnosed breast cancer has been controversial. The clinical outcome literature on the benefits of preoperative MRI shows conflicting results regarding rates of re-excision and local recurrence.
A randomized controlled trial conducted in the United Kingdom known as the COMICE trial, published in 2010, compared two patient groups: one group received preoperative MRI and the other group received standard care. Re-excision rates were identical (19%) for both the MRI and non-MRI groups. 39 This trial was widely criticized, partly because older MRI technology was used across the imaging sites, and partly because radiological and surgical experience was varied. Another problem with this trial involved the lack of MR-guided biopsy procedures, which were not generally available at the trial sites. This deficiency resulted in management changes to mastectomy in 28% of the MRI group, who went to surgery without presurgical confirmation of malignancy. The MONET trial found an increase in re-excision rates in patients with MRI (34%) versus for patients without MRI (12%). This paradoxical higher rate of positive margins and re-excision rates in the MRI group was attributed to surgical bias; critics of this study noted that, overall, the volume of the excised tissue in the MRI group (69.1 cm3) was much smaller than the volume in the non-MRI group (90.2 cm3). Additional variability was noted in surgical resection volumes, which were even smaller in patients with DCIS and negative MRI examinations (40.3 cm3). 40 A later study in 2012, where biopsy confirmation of malignancy was made prior to management change, found a significant improvement in reoperation rates using preoperative breast MRI. Patients in the MRI group in this study had a reoperation rate of only 11% compared to 25% in the non-MRI group, without an increase in the mastectomy rates, 41 and recently published studies have shown decreased rates of re-excision when preoperative MRI was used. 42 , 43 The data on the efficacy of use of preoperative MRI for patients with invasive lobular carcinoma (ILC) lesions are particularly convincing. 44 , 45 , 46
It is important to note that use of reoperation rates as a metric for clinical efficacy of preoperative MRI is confounded by the wide variation in surgical practice, which may override any impact that MRI findings might make. 1 There is considerable inconsistency in recommendations for preoperative MRI in the United States. Some surgeons and oncologists request MRI before surgery or neoadjuvant chemotherapy routinely, while others may select preoperative MRI for younger women only or for those with ILC histology, and others do not recommend preoperative MRI. Several studies have documented the critical role of preoperative MRI for women selected for partial breast irradiation. 47 , 48 No survival benefit has been demonstrated until now. A prospective randomized controlled trial is currently under development by the American College of Radiology Imaging Network (ACRIN) on the short- and long-term benefits and cost analysis of preoperative MRI staging. 49
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree