10 Pelvic and Gonadal Venous Reflux
Summary
Pelvic and gonadal venous reflux syndromes are underdiagnosed and undertreated pathologic entities. As demonstrated in this chapter, reflux and obstruction of the gonadal and pelvic veins are significant causes of morbidity in men and women. Minimally invasive, percutaneous image-guided therapies offered by interventional radiologists are efficacious for these syndromes, and are characterized by low periprocedural complications and rapid recovery. This chapter describes the epidemiology of these disorders, symptom complex, and patient presentation, with details of patient evaluation and development of a treatment plan. Included in the chapter are procedural details to assist the new or experienced interventional radiologist in managing this patient population, and the text describes a plan for follow-up after a therapeutic intervention.
10.1 Introduction
Pelvic and gonadal venous reflux syndromes are poorly understood in the general medical community, leading to misdiagnosis or frequent delays in diagnosis. The central underlying pathologic process that unifies this group of disorders is pelvic venous hypertension caused by primary pelvic venous insufficiency and/or mechanical venous obstruction. Chronic pelvic venous hypertension can lead to dilation and engorgement of various venous collateral pathways within the pelvis. The clinical manifestations of each syndrome are specific to the particular venous structures affected and can significantly impact a patient’s quality of life, psychosocial wellbeing, and reproductive health. Consequently, a substantial economic impact is realized when large populations are considered. Herein, pelvic and gonadal venous reflux will be discussed with a focus on pelvic congestion syndrome, varicocele, and nutcracker syndrome.
10.2 Pelvic Congestion Syndrome
Pelvic congestion syndrome (PCS) is defined by chronic pelvic pain (CPP) lasting longer than 3 to 6 months, which is caused by incompetent or obstructed gonadal veins and/or pelvic branches of the iliac venous system. 1 The precise mechanism by which venous congestion causes pain in PCS remains somewhat unclear but is likely multifactorial. 1 Historically, the earliest reports of PCS date to 1831 by Gooch and subsequently in 1857 by Richet as a “tubo-ovarian varicocele.” 2 , 3 In 1928, Cotte further elaborated on the phenomenon of impaired circulation and drainage leading to enlarged venous complexes involving the reproductive tissues. 4 The effect on the female reproductive organs was described in 1949 by Taylor, correlating the dilated pelvic veins with symptoms of CPP, which was ultimately established by Beard et al in 1984. 5 , 6
Presently, between 30 and 40% of women will suffer from CPP throughout their life. Additionally, 10 to 15% of asymptomatic women have radiographically demonstrable pelvic varices, which arise from the ovarian, internal iliac, or parauterine veins. 7 While both pelvic pain and venous varices are often present in multiparous women of childbearing age, they may not be related. As such, accurately discriminating PCS from alternative etiologies of CPP can be challenging. A thorough workup with history and physical examination, laboratory results, and imaging studies can narrow the differential diagnosis.
10.3 Case Vignette
10.3.1 Patient Presentation
A 25-year-old woman presents with a primary complaint of pelvic pain, describing it as a dull heaviness that worsens over the course of the day. The pain has been ongoing for several months and worsens before menstruation and during/after intercourse. She is otherwise healthy with a negative review of systems and negative gynecologic workup. With the exception of two pregnancies with normal spontaneous vaginal deliveries, she has no past medical, surgical, or pertinent family history.
10.3.2 Physical Exam
Notable for tenderness at the ovarian points bilaterally and superficial vulvar varicosities.
10.3.3 Imaging
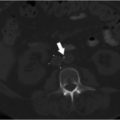
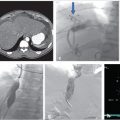
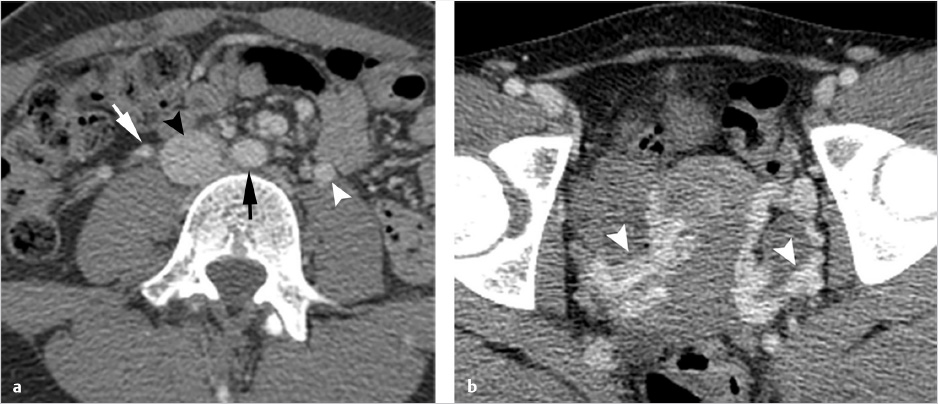
Ultrasonography reveals multiple tortuous vessels around the uterus, which increase in size with Valsalva’s maneuver (Fig. 10.1a,b). Pelvic CT reveals an enlarged left gonadal vein, prominent parauterine veins, and vulvar varicosities (Fig. 10.1c). Pelvic MRI reveals retrograde flow in the left gonadal vein (Fig. 10.1d).
10.3.4 Specifics of Consent
The procedure and technical approach, whether from internal jugular or femoral veins, is presented. In addition to reviewing potential complications associated with moderate sedation, venography, embolization, and sclerotherapy, it is important to set appropriate expectations for procedure outcomes and the potential need for additional treatment of internal iliac veins, if not treated concurrently, or superficial varicose veins. Lastly, discuss the available evidence and note that there are no data to support a negative effect on ovarian function, menstruation, or fertility.
10.3.5 Details of Procedure
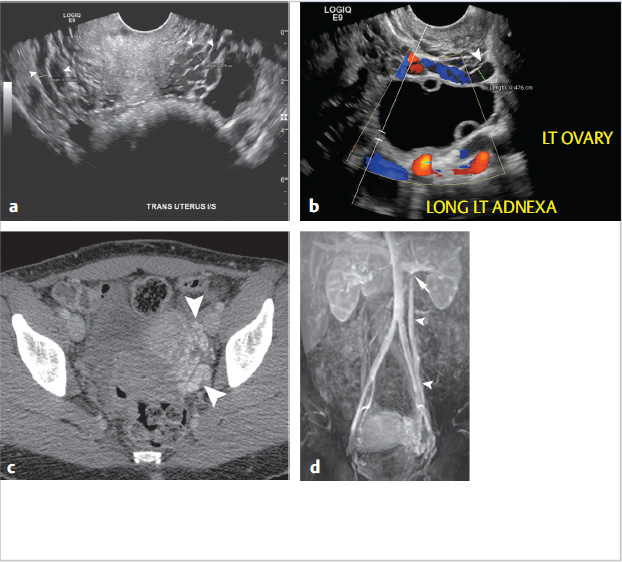
Through a right internal jugular approach, the left renal vein (LRV) was selected for venography (Fig. 10.2a), after which the left gonadal vein was selected. Left gonadal venography demonstrated retrograde flow into the pelvic varices (Fig. 10.2b). The catheter was advanced into the pelvis, and after venography, sclerotherapy was performed utilizing foamed sclerosant (equal parts 3% sodium tetradecyl sulfate and iodinated contrast, agitated with air through a three-way stopcock) “sandwiched” with mechanical occlusion (Fig. 10.2c).
10.3.6 Follow-up
For most patients, this is an outpatient procedure since it is not associated with significant postprocedural symptoms beyond mild pain and nausea. Approximately 1 week after the procedure, the patient returned for an outpatient clinic visit to evaluate for early complications, review the procedure, and answer any questions. Routinely, a 3-month return visit is scheduled for reevaluation of symptoms and to determine procedural success or need for further intervention.
10.4 Epidemiology and Scope of the Problem
CPP in women is a significant global health issue, which affects an estimated 2 to 24% of all women worldwide (according to a published review from the World Health Organization in 2006). 8 , 9 In the United States, it is estimated that approximately 30% of all outpatient gynecological visits are specifically for the evaluation of CPP. 10 The economic burden of diagnosing and treating CPP is estimated at $1.2 billion annually, with an additional $15 billion lost due to lack of economic production. 11 Of those suffering from CPP, 30% are thought to be secondary to PCS. According to a 2014 U.S. census, there are 63 million women in the United States between the ages of 25 and 53, which should, presumably, equate to an incidence of approximately 5 to 6 million women suffering from PCS. In addition to the micro- and macroeconomic burden, these patients frequently suffer a significant psychological toll as increased levels of anxiety, stress, and depression have all been liked to PCS. 10
10.4.1 Patient Presentation and Evaluation
Often, PCS patients visit several physicians and are misdiagnosed before presenting for endovascular therapy. Unfortunately, the differential diagnosis of CPP in women is extensive. Excluding alternative etiologies is paramount, and the differential diagnosis can be narrowed with a thorough history and physical examination, laboratory exams, and imaging.
Most commonly, patients are multiparous, premenopausal, and suffer from several months of pelvic heaviness or a dull diffuse ache. Symptoms may reproducibly worsen with prolonged standing and improve while recumbent. The pain should not be strictly cyclical or only coinciding with menses. Patients may also describe dyspareunia or postcoital pain if vulvar or vaginal veins are involved. Bladder and bowel symptoms can also be present, including urinary frequency, urinary hesitancy, and symptoms similar to irritable bowel syndrome. 1
On physical exam, ovarian point tenderness may be elicited. In a study by Beard et al, the combination of ovarian point tenderness on physical exam with postcoital pain was 94% sensitive and 77% specific for diagnosing PCS, though these findings have not been reproduced to date. 12
Vulvar, perineal, and upper thigh varicosities can be visualized, and made more prominent with the patient standing or performing a Valsalva maneuver. Since PCS remains a diagnosis of exclusion, the caregiver should pursue additional imaging if any combination of the above symptoms or physical exam findings is present.
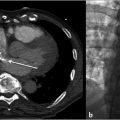
Imaging is performed in order to document the characteristic pelvic venous changes of PCS, which support the diagnosis but do not define it. Imaging is also performed in order to exclude other causes of CPP. Traditionally, ultrasound (US) has been widely regarded as the modality of choice; characteristic findings include dilation of the ovarian veins (>4 mm in diameter), slow (<3 cm/s), or retrograde flow in the ovarian veins that may worsen with Valsalva, presence of tortuous and dilated pelvic venous plexuses, dilated arcuate veins (>5 mm) crossing the uterine myometrium and communicating with the pelvic venous plexuses, and polycystic changes of the ovary. 13 An additional advantage of US is the ability to perform the examination in the semi-supine or upright position, as some patients’ symptoms and venous dilation will remit in a fully supine position. Time-resolved pelvic magnetic resonance imaging (TrMRI) has recently been shown to accurately demonstrate pelvic venous reflux while simultaneously providing excellent anatomic detail of the pelvis (Fig. 10.3). However, evaluation of the vasculature by TrMRI is slightly limited in that it requires the patient to lie in a supine position, and it is less cost-effective when compared to US. Computed tomography venography (CTV) can be of some benefit as it can provide excellent anatomic vascular detail. However, CT is unable to produce the requisite dynamic information necessary to diagnose venous reflux, which limits its utility in PCS. On the other hand, CT can be beneficial in the general workup of CPP secondary to its ability to provide anatomic detail and quick acquisition times (Fig. 10.4a,b). Direct venography (DV) is classically the test of choice for diagnosing venous reflux; however, given the invasive nature of venography and the excellent accuracy of US, DV has been largely replaced by US in the diagnostic setting.
The American Venous Forum has published clinical practice guidelines, which address varicose veins and chronic venous disease. 14 At present, noninvasive imaging with US, CT, and MRI receives the highest grade of recommendation with retrograde ovarian and iliac venography reserved for patients in whom an intervention is planned. Ultimately, patients with a suspicious clinical history and concordant imaging findings of pelvic/gonadal venous insufficiency should be considered for treatment, assuming alternative diagnoses of CPP have been excluded. Additionally, if a patient has a compelling history and physical without correlative imaging findings, a definitive diagnosis can be pursued with semi-upright venography or with Valsalva at the time of venography.
10.4.2 Preparation for Procedure
After the clinic visit, which should include reviewing patient clinical status, allergies, labs, and imaging, no other specific preprocedural testing is needed. Preprocedural medications may include a single dose of an antibiotic to cover skin flora, but no other medications are necessary. Nursing should acquire intravenous access that can be used for the administration of moderate sedation as needed, fluids for hydration, and antiemetics. General anesthesia is not required unless there are comorbid conditions that warrant it. Placement of a urinary catheter is also not required.
The patient is positioned supine in an interventional suite, and sterile preparation and draping is performed for the access site, commonly the right internal jugular and/or right common femoral vein. A tilt table may be helpful to accentuate the findings on venography, but is not required. No special equipment is needed beyond the standard repertoire found in most interventional radiology suites. As with any endovascular procedure, the operator should be mindful of contrast volume and radiation dose. Fluoroscopic image saves should be utilized in lieu of standard digital subtraction runs during the endovascular procedure, if possible. These radiation safety measures are particularly important for this patient population, many of whom are still of childbearing age.
10.4.3 Technical Tips and Tricks and Procedural Details
Preferred access site is the right internal jugular vein, since this allows evaluation and possible treatment of the bilateral gonadal and the bilateral internal iliac veins. However, these veins may all also be selected from a common femoral approach. The approach to selection and treatment of the gonadal veins and internal iliac veins is slightly different, and each is discussed separately.
Gonadal Vein Embolization
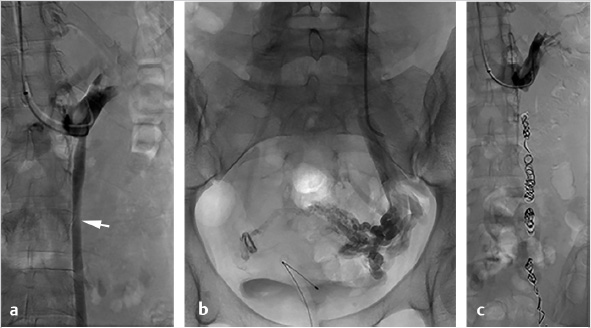
A 5- to 7-French vascular sheath is placed at the access site, through which a 4- to 5-French angled tip catheter is used to gain access to the veins. The left gonadal vein is usually larger and more easily accessed than the right gonadal vein. Care should be taken to minimize potential spasm or injury to the veins during selection. The left gonadal vein arises from the LRV, which is selected for venography to demonstrate incompetence of the gonadal venous valves. Subsequently, the left gonadal vein is selected with a guidewire, and the catheter is advanced into the pelvis, close to the varicosities, for additional venography (Fig. 10.5a). In contrast to the left, the right gonadal vein terminates directly as a confluence with the inferior vena cava (IVC), and can be accessed with an angled catheter from the jugular approach or a reverse-curve catheter, such as a Simmons 1, from a femoral approach. As with the left, the catheter is navigated into the pelvis, close to the varicosities, for additional venography. If there is difficulty advancing a 4- or 5-French catheter, a coaxial microcatheter (2.4–2.8 French) can easily reach deep into the pelvis.
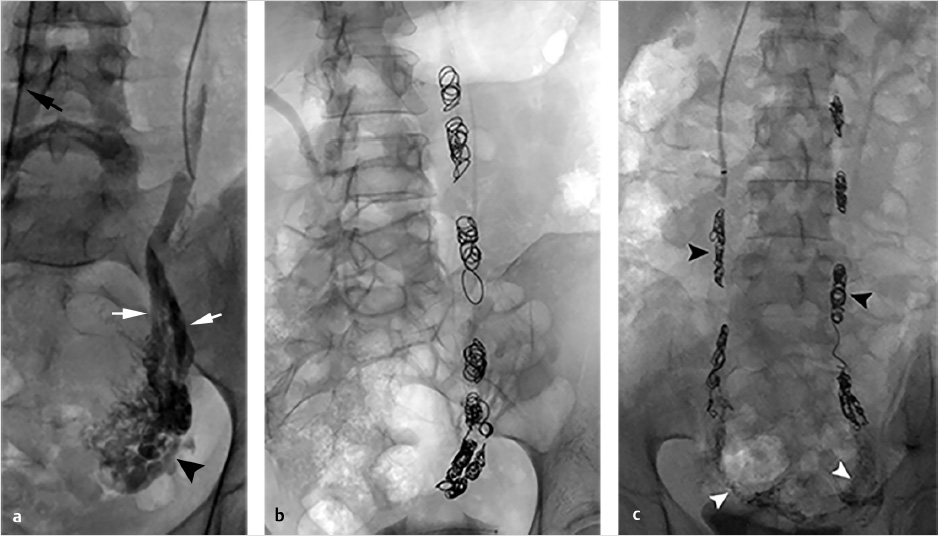
Once positioned in the pelvis and following venography, adjacent to the varicosities, embolization can be performed with a combination of coils, plugs, and sclerosants. Sclerosants can be either liquid or foamed, and, when used, allow for deeper penetration of the treatment area. The use of occlusion balloons is dependent on operator preference. Embolization is performed from the upstream (most caudal) pelvic veins in ascending fashion to the termination of the gonadal vein. Sclerosant is alternated with mechanical occlusion every few centimeters, which creates an embolic “sandwich” allowing for complete embolization while additionally helping to prevent collateralization of the reflux circuit (Fig. 10.5b).
Internal Iliac Vein Embolization
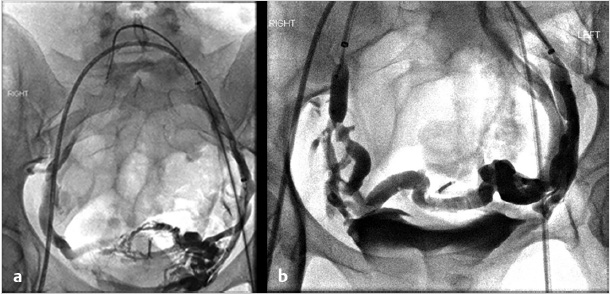
If symptoms are refractory after treatment of the gonadal veins, the potential for internal iliac venous incompetence should be assessed. Some operators choose to evaluate internal iliac vein incompetence during a separate procedure, while others treat both the gonadal and internal iliac veins on the same day without consequence. After selecting each internal iliac vein, venography is performed (Fig. 10.6a). Balloon occlusion venography is performed to better visualize the varicosities (Fig. 10.6b); in the absence of balloon occlusion, the brisk inflow into the circulation may not allow visualization of the multiple draining veins. With balloon occlusion, the tributary veins are visualized with test injections prior to subsequent balloon-occluded sclerotherapy. After a dwell time of approximately 10 minutes and with fluoroscopic monitoring, the occlusion balloon may be deflated. This is repeated on both sides, or may be done concurrently with bilateral access. Mechanical occlusion of the internal iliac veins has been previously reported, and is performed in some practices, depending on operator preference.
10.4.4 Potential Complications or Pitfalls
Major complications are uncommon and can include nontarget embolization of coils or plugs to the pulmonary circulation. This can be prevented by using detachable plugs or coils at the rostral portion of the ovarian vein and by oversizing the mechanical embolics by 30 to 50% to account for venous distensibility due to patient volume status and supine intraprocedural positioning. Migration of the coil or plug can be treated with snaring and removal if necessary. Other complications may include vessel perforation, pelvic venous thrombophlebitis, pelvic pain, and recurrence of varices. The overall reported rates for all complications are less than 4%. 9 , 13 , 15 , 16
10.4.5 Postprocedural Management and Follow-up
Patients are observed for approximately 2 to 4 hours postprocedure in order to recover from sedation. Pain management, antiemetics, and intravenous hydration are all important components of postprocedural care. Patients are discharged with prescriptions for pain medications and antiemetics, though ibuprofen and naproxen are encouraged as a first-line therapy. Transient pelvic pain, particularly if sclerosants are used, is not unusual and may represent a degree of phlebitis. Generally, this will subside in a few days and can be managed conservatively. A minority of patients may require overnight admission for debilitating pain. 17 , 18 , 19
Normal activity may be resumed after 72 hours. No special labs or unique monitoring is required in the postprocedural setting. Postoperative pain scores should be obtained and compared with preoperative scores, though pain improvement times can vary widely. The literature suggests that immediate improvements should not be expected, as the earliest reports of pain improvement occur at 1 week after the procedure but may lag for up to 3 months postprocedure. An early follow-up, in the range of 2 weeks, is performed in order to assess for complications, review the procedure and expectations, and monitor recovery. A second clinic visit is scheduled at approximately 12 weeks to reassess pelvic pain and to determine if additional treatment is warranted. Afterward, patients may return as needed on an annual basis or earlier if symptoms recur. These visits include standardized pain assessments, and specifically address overall pain, pain on standing, pain on lying down, dyspareunia, menstrual pain, and urinary alterations. Repeat imaging is not routinely performed and should be pursued only if symptoms warrant further evaluation.
10.4.6 Outcomes
Ovarian vein embolization for PCS was first described in a case report in 1993. 20 Subsequently, multiple observational case series and meta-analyses have been published describing the technique and outcomes. These studies were summarized in a recent meta-analysis by Mahmoud et al, which demonstrated an overall immediate technical success rate of 99%, improvement in symptoms categorized by the patients as “significant improvement” in 88.1% at 1 to 3 months postprocedure, and 86.6% long-term symptomatic relief. Follow-up times ranged from 7 months to 5 years. 21 Finally, the rate of ovarian vein recanalization requiring repeat embolization is estimated at approximately 2 to 4% based on the largest available series. 19 , 21
10.5 Pearls of Wisdom
Patient evaluation and exclusion of alternative etiologies of CPP is paramount to increase technical and clinical success of embolization procedures.
Treatment of the entire length of the ovarian vein helps prevent collateralization of retroperitoneal veins to the refluxing pelvic veins.
Using sclerosants, in addition to mechanical occlusion devices, can more deeply ablate interconnected pelvis venous plexuses.
PCS has a complex etiology that requires multimodality treatment. Endovascular embolization and sclerotherapy are successful in eliminating the physical cause, but psychosocial supportive therapy and medical treatment may also be necessary to achieve the best results for patients.
10.6 Unanswered Questions
PCS continues to be a misunderstood and underdiagnosed cause of pelvic pain. Timely diagnosis and prompt endovascular treatment can effectively mitigate the somatic, psychological, and economic consequences of the disease. While large, prospective randomized trials are lacking, emerging case series and meta-analyses suggest high rates of clinical success and low rates of complications when patients undergo endovascular embolization. Prospective trials comparing medical management and invasive therapies would be helpful to delineate which management strategy would best serve this diverse patient population and provide convincing outcomes data. Moreover, refinement of the diagnostic imaging criteria, investigations of optimal embolic choice and technique, and outcomes data comparing different techniques (unilateral vs. bilateral ovarian vein embolization, and ovarian vein vs. combined ovarian and internal iliac vein embolization) could further guide practitioners and optimize patient care.
10.7 Varicocele
The first description of varicocele is attributed to Celsus, a Greek physician in the first century, who described veins that were “swollen and twisted over the testicle.” 22 Currently, varicoceles are defined as an abnormal distension of the pampiniform venous plexus and are the male equivalent of PCS. Like PCS, it is thought to be a result of gonadal vein incompetence, which may be from primary venous insufficiency or secondary to venous compression/obstruction. Varicoceles are a common cause of scrotal pain, predispose to infertility, and may arrest testicular growth in adolescents and adult males that may lead to decreased testosterone levels. 23
The connection between varicocele and male infertility dates back to the late 19th century, but documentation of improved semen parameters and subsequent successful pregnancies after treatment were not reported until 1955. 24 , 25 The ultimate goal of treatment relies on the disruption or occlusion of the dilated veins that drain the testis. Currently, in North America urologic microsurgery is the most commonly employed treatment technique, but a growing body of evidence suggests that percutaneous embolization is a safe alternative offering the potential of improved patient safety, lower morbidity (hydroceles and impotence), and lower costs. 24 , 26 , 27
10.8 Case Vignette
10.8.1 Patient Presentation
An otherwise healthy 29-year-old man initially presented to his urologist for infertility. The patient complained of left testicular pain that was exacerbated by prolonged periods of sitting. His review of symptoms and past medical history were otherwise negative.
10.8.2 Physical Exam
The patient was noted to have a grade III left-sided varicocele, which was visible without Valsalva.
10.8.3 Noninvasive Testing
Semen analysis revealed slow or sluggish progressive sperm motility despite an adequate sperm concentration.
10.8.4 Imaging
Scrotal US identified anechoic tubular structures with vascular flow adjacent to the left testis. The vessels measured greater than 3 mm in size and enlarged with Valsalva’s maneuver. The right side was unremarkable.
10.8.5 Specifics of Consent
The procedure and technical approach, whether from internal jugular or femoral veins, is presented. In addition to reviewing potential complications generally associated with moderate sedation, venography, embolization, and sclerotherapy, setting appropriate expectations for procedure outcomes and the potential for continued difficulty conceiving (clinical failure) is important.
10.8.6 Details of Procedure
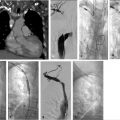
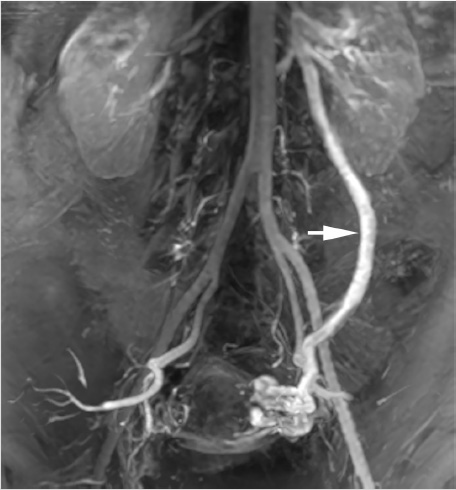
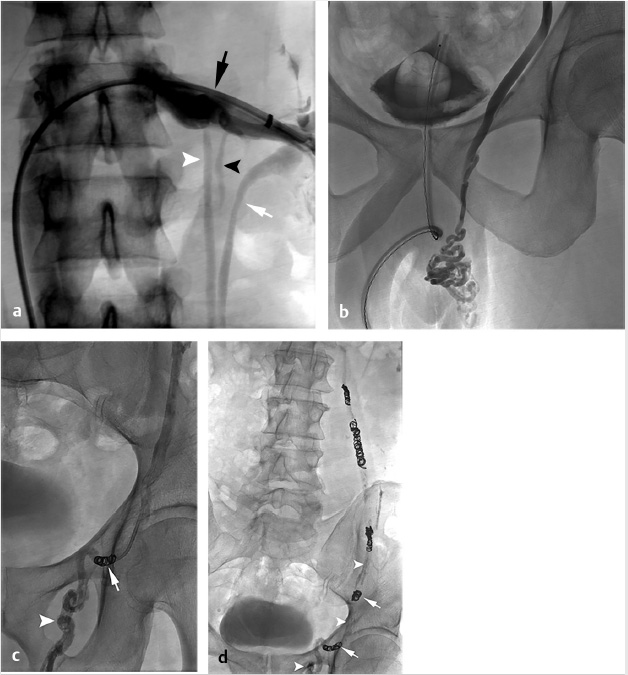
The procedure was performed via the right femoral vein using a long, hydrophilic 6-French sheath (although internal jugular access could have been used as well). A left renal venogram was performed on a tilt table positioned in reverse Trendelenburg 20 degrees, with a catheter beyond the orifice of the left gonadal vein and the patient performing a Valsalva maneuver (Fig. 10.7a). After confirmation of retrograde flow into the gonadal vein, the catheter was advanced to the midleft gonadal vein and repeat venography was performed, confirming the varicocele (Fig. 10.7b). Next, a combination of coils and sclerosant (3% sodium tetradecyl sulfate solution mixed with contrast) were used for embolization and sclerosis. Some operators put an emphasis on placing coils most distally to avoid deposition of sclerosant into the pampiniform plexus. However, some believe that improved efficacy may be achieved with deposition of sclerosant into the varicocele (Fig. 10.7c,d). A prominent right gonadal vein could not be visualized.
10.8.7 Follow-up
The immediate postprocedure course was uneventful. On 3-month follow-up, the patient no longer had a varicocele and the couple was able to conceive. A scrotal US at that time was unremarkable.
10.9 Epidemiology and Scope of Problem
Varicoceles are present in 15 to 20% of young, healthy, asymptomatic men, but the prevalence increases with age, reaching as high as 42% in the elderly population. However, the incidence of a varicocele in men who present for evaluation of infertility is disproportionately high (35–40%). 28 While the majority of males with a varicocele are asymptomatic, there is a growing body of evidence linking varicoceles with progressive decline in testicular function; this culminates in impaired semen parameters, infertility, and decreased serum testosterone levels. 29
According to the World Health Organization, infertility is defined by the inability of a sexually active couple to conceive after 1 year of unprotected intercourse 30 ; it is estimated that 15% of all couples are infertile under this definition. Further, male factors are solely responsible for the couples infertility in about 20% of cases and a contributing factor in an additional 30 to 40% of cases. 31 Most cases of infertility are idiopathic, but varicoceles are the most commonly identified cause of male factor infertility, accounting for 15% of all cases. When only azoospermic patients are considered, varicoceles are the second most common underlying diagnosis, behind maldescended testes (10.9 and 17.2%, respectively). 32 , 33
While most varicoceles are asymptomatic, 2 to 10% are associated with pain; this equates to an estimated 35 million men worldwide. 34 , 35 In fact, varicoceles have been reported to be the cause of pain in 2 to 14% of all men suffering from chronic scrotal pain. 36 Much like its female counterpart, PCS, chronic scrotal pain can significantly impact the daily activities of those suffering from this entity and can lead to significant psychosocial issues.
10.9.1 Patient Presentation and Evaluation
Men with a varicocele present with a variety of clinical scenarios, and treatment of each has unique therapeutic goals and diagnostic workup. Thus, proper patient management is tailored to the presenting clinical situation. The three primary indications for intervention are infertility, testicular atrophy in the adolescent or pediatric patient, and pain. Clinical evaluation begins with a history, physical examination, and scrotal ultrasonography.
Most often, varicoceles are asymptomatic and patients present for infertility. According to the American Urological Association (AUA) and the American Society for Reproductive Medicine (ASRM), routine evaluation of infertile men with a varicocele should include a medical and reproductive history, a physical exam, and a minimum of two semen analyses. 37 , 38 On physical exam, upon palpation the clinician may appreciate a “bag of worms” within the scrotum, irrespective of the presence or absence of pain. Physical exam is best performed with the patient in the standing position, and a Valsalva maneuver should be performed during the exam in order to accentuate dilation of the pampiniform plexus and properly grade the varicocele. 23 Varicoceles are then classified as Grade I (palpable only with Valsalva), Grade II (palpable without Valsalva), and Grade III (visible from a distance).
Treatment of a varicocele in the setting of infertility is advocated when the following criteria are met in the adult male: the couple has known infertility; the female partner has been evaluated and has no identifiable abnormality or has a potentially treatable cause of infertility; the varicocele is palpable on physical examination; and the male partner has abnormal semen parameters or abnormal results on sperm function tests. Treatment should also be offered to any adult men who are not currently attempting to conceive but desire future fertility, have a palpable varicocele, and abnormal semen analyses. Of note, the AUA and the ASRM do not currently recommend treatment in adult men if semen analysis is normal or the varicocele is not palpable (i.e., “subclinical”), pointing to the fact that “only palpable varicoceles have been documented to be associated with infertility.” 37 Given the controversial link between varicoceles and reduced ipsilateral testicular size and function, the AUA and ASRM suggest that treatment should be “considered” in adolescent males with unilateral or bilateral varicoceles and with objective evidence of reduced ipsilateral testicular size. 37 These associations suggest annual follow-up with US to document testicular size and/or annual semen analysis in order to detect the earliest sign of varicocele-related testicular dysfunction.
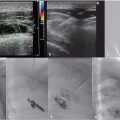
Scrotal US with pulsed and color Doppler images is an invaluable tool in the evaluation of a suspected varicocele (see Fig. 10.8a–c). On grayscale US, the dilated pampiniform venous plexus is apparent as serpiginous, tubular-shaped anechoic structures. Threshold vein diameters of 2.5 mm at rest and 3.0 mm during Valsalva have been shown to be over 80% predictive of clinically-apparent varicoceles (Grades I-III). 39 Varicoceles are noted on US, but those that are occult on physical exam are categorized as Grade 0 or “subclinical.” It is worth noting that the AUA and the ASRM have both recommended reserving US for those cases of inconclusive physical examination in the setting of infertility; however, some authors have advocated for the use of US in the evaluation of male infertility and pediatric varicoceles. These authors argue that US can be used to follow pre- and posttreatment measurements as a measure of clinical success and to evaluate for postoperative complications such as hydrocele or testicular atrophy secondary to inadvertent testicular artery ligation. 23 , 32 , 37 , 38 , 40
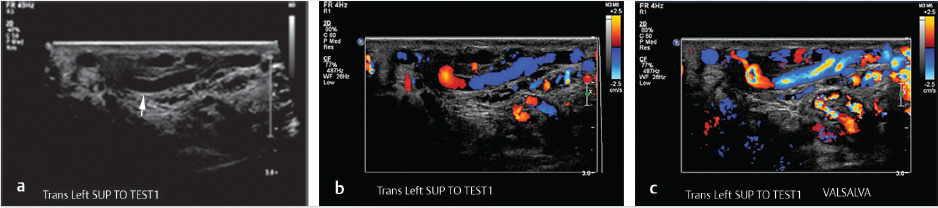
Patients presenting with pain describe a “dull” and/or “throbbing” scrotal pain that extends to the ipsilateral inguinal region. Much like its PCS counterpart, the pain associated with a varicocele tends to worsen with straining, exercise, and long periods of standing. 35 US evaluation in these cases can exclude alternative diagnoses and confirm the diagnosis of varicocele as the sole abnormality. A new, right-sided unilateral varicocele is concerning for an intra-abdominal mass and cross-sectional imaging and evaluation is recommended. No specific criteria exist for the treatment of varicocele in the setting of pain, provided that the history, physical exam, and imaging findings are concordant.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree