10 SPECT in Children
10.1 Introduction
Soon after the availability of single-photon emission computed tomography (SPECT), it was used for imaging children. 1 As SPECT cameras became distributed more widely in the 1980s, pediatric applications became similar to those in the general population, although the clinical utility of SPECT in pediatrics has reflected the distribution of disease in children. Some SPECT studies are more likely to be performed in children: renal cortical imaging, splenic imaging, and tumor imaging with metaio-dobenzylguanidine (MIBG); these will be the focus of this chapter.
SPECT is used for many other studies in pediatric nuclear medicine and may be performed for indica tions more common in children. For example, in children and young adults, skeletal scintigraphy may be performed for sports medicine indications (see Chapter 8). For other procedures such as brain SPECT or parathyroid SPECT, the indications and procedures in children are similar to those in adults (see Chapters 3 and 4). Other SPECT studies, such as myocardial perfusion imaging (see Chapter 5), are performed less frequently in children than in adults.
As in adults, the benefits of SPECT in children include improved lesion conspicuity due to greater image contrast 2 and the availability of cross-section al images that can be coregistered with computed tomography (CT) and magnetic resonance imaging (MRI). However, in some pediatric situations, SPECT does not have a benefit over planar scintigraphy. In small children, the width of the camera bed may prevent rotating camera heads from being in close proximity to the organ(s) of interest. Thus, in children less than 1 year of age, planar images acquired with a pinhole collimator may provide more informative images than can be acquired with SPECT. The utility of hybrid SPECT/CT in pediatrics remains poorly defined, in part, due to concerns about radiation dose. On the other hand, newer techniques of SPECT image reconstruction 3 can be of particular interest in pediatrics. These methods of image processing can improve image quality while facilitating use of a smaller administered activity of radiopharmaceutical so that diagnostic accuracy can be improved while radiation dose is decreased.
10.2 Renal Cortical SPECT with 99mTc-DMSA
10.2.1 Indications
Renal cortical SPECT has a broad range of indications, mostly of particular interest in children and young adults.
Cortical renal scintigraphy provides an accurate determination of differential renal function and can be an important part of the evaluation of patients with renal or urologic diseases.
Renal SPECT can be used to identify functional cortical lesions, including scars, cysts, and dysplasia, and it has an important and evolving role in the evaluation of both acute pyelonephritis and the possible sequelae of inadequately treated kidney infections.
Occasionally, cortical scintigraphy can be helpful in localizing a kidney in patients with a possible ectopic (e.g., pelvic) or congenitally absent kidney.
Correlation with renal ultrasound can be very helpful when interpreting renal SPECT, but coregistration of SPECT and CT images is rarely necessary.
10.2.2 Technique
Radiopharmaceutical
Cortical renal scintigraphy is performed with technetium-99 m dimercaptosuccinic acid (99mTc-DMSA). After administration by intravenous injection, nearly all (90%) 99mTc-DMSA becomes protein bound 4 and clears from the blood pool with a half-life of slightly less than 1 hour. 5
Renal cortical uptake is approximately 40 to 50% by 1 hour and 70% by 24 hours, 4 , 5 with most 99mTc-DMSA accumulating in the epithelial cells of the proximal convoluted tubules. 6
The current harmonized guidelines recommend an administered activity of 1.85 MBq/kg (0.05 mCi/kg), with a minimum of 11.1 MBq (0.3 mCi) and a maximum of 111 MBq (3.0 mCi). 7
Imaging
Cortical scintigraphy is typically performed 2 to 4 hours after radiopharmaceutical administra tion. Uptake of 99mTc-DMSA is limited to the renal cortex, with no accumulation in the medulla; therefore, the pattern of 99mTc-DMSA uptake demonstrates the structure of the normal renal cortex.
Although most 99mTc-DMSA is retained by the renal cortex, a small amount is excreted into the renal collecting system. In patients with substantial collecting system obstruction, trac er accumulation in the collecting system may interfere with imaging. In these patients, improved diagnostic accuracy and quantitation may be obtained by waiting even longer (up to 24 hours) before performing cortical scintigraphy. 8
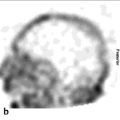
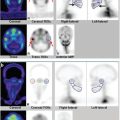
Renal cortical scintigraphy can be acquired as static planar images or with SPECT. Planar renal scintigraphy is performed either with parallel-hole collimators or with pinhole collimators, which will provide a magnified and more detailed image of the kidney than available with planar parallel-hole collimators. However, pinhole images can be acquired in only limited projections (typically posterior and posterior oblique). Renal cortical SPECT using high-resolution or ultrahigh-resolution parallel-hole collimators provides higher quality images of the renal parenchyma than planar images acquired with either a parallelhole or a pinhole collimator. 9 After reconstruction, SPECT can be displayed in three planes (transverse, coronal, and sagittal) or as rotating volume-rendered maximum intensity projection images (► Fig. 10.1). These two methods of image representation can be complementary when trying to identify and characterize lesions in the renal cortex.

Differential Renal Function
The relative distribution of tracer between the kidneys reflects differential renal blood flow and the mass of functional renal cortex. 10 , 11 Differential function is determined using regions of interest or volumes of interest to define renal cortex and background soft tissue. Separate regions can be applied to each moiety of a duplicated kidney. Accurate determination of renal size requires image-size calibration.
10.2.3 Pyelonephritis
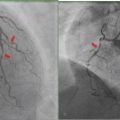
Pyelonephritis is a urinary tract infection (UTI) involving the renal parenchyma. With appropriate therapy, pyelonephritis can resolve with no longstanding sequelae. However, delayed therapy can result in permanent renal cortical damage, which may increase the future risk of hypertension or chronic renal failure. 12 Renal scarring can be prevented by early antibiotic therapy. 13 , 14 , 15 , 16 Cortical renal SPECT can be used for both the initial evaluation of acute pyelonephritis and follow-up after resolution of the kidney infection (► Fig. 10.2).
When used to evaluate patients with febrile UTI, 99mTc-DMSA scintigraphy has very high sensitivity and specificity for the early diagnosis and localization of cortical involvement, 17 , 18 , 19 , 20 , 21 particularly when imaged with SPECT. 9 Sites of infection will have decreased uptake of 99mTc-DMSA, and typically, during the acute infection, these cortical lesions will have poorly defined margins. Occasionally, focal sites of infection may appear to have increased volume compared to the normal renal cortex.
If appropriate therapy is started (usually within approximately 48 hours) and there is successful treatment of the infection, the cortical abnormalities typically resolve with normalization of the scintigraphic abnormalities within 3 to 6 months, although long-term damage may be possible. 13 However, it is not clear that all renal cortical defects will persist and therefore have a clinical effect. 22 Rarely an infection may involve the entire renal parenchyma, which will appear as decreased 99mTc-DMSA uptake throughout the whole kidney.
In the absence of prompt and appropriate therapy, a renal infection will produce permanent cortical damage or scarring. 13 , 14 , 15 On follow-up 99mTc-DMSA scintigraphy, regions of scar demonstrate clearly defined sharp margins with a corresponding loss of cortical volume. The typical cortical scar may appear wedge-shaped, although the appearance will depend on the location and age of the scar, as well as the degree of growth by the surrounding normal cortex.

10.2.4 Approaches to Imaging Recurrent Urinary Tract Infection
The role of renal cortical scintigraphy in the evaluation of recurrent UTI and suspected vesicoureteral reflux has evolved over the past decade. 23
Until recently, at most centers in North America, a child with a febrile UTI would be evaluated by the “bottom-up” approach. 14 The initial step has been an evaluation for possible vesicoureteral reflux. Detailed evaluation of the renal parenchyma was reserved for patients with repeated infections or breakthrough infections while on antibiotic prophylaxis.
More recently, the “top-down” approach has gained favor among clinicians. 24 With this approach, the initial evaluation of a febrile UTI is focused on the kidney, and evaluation for reflux is performed only in patients with demonstrated renal involvement of the infection. There is variability in how the top-down approach has been implemented. Ideally, the top-down approach starts with renal cortical scintigraphy within 7 to 10 days of the febrile urinary infection. If evaluation is delayed, then signs of acute cortical infection may have resolved, and delayed imaging can give false reassurance that evaluation for vesicoureteral reflux is not needed. 24
Although renal cortical scintigraphy is more sensitive for identifying renal cortical injury, 19 , 25 some recent guidelines have allowed the use of renal ultrasound and have recommended that evaluation be performed only in patients with more than one febrile UTI. 15 However, these guidelines have been controversial because of concern that clinically important renal pathology or scarring will be missed. 26 , 27 , 28
10.2.5 Morphological Renal Abnormalities
99mTc-DMSA cortical renal SPECT can have utility in the identification and characterization of morphological renal abnormalities.
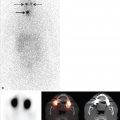
Renal SPECT can be complementary to renal ultrasound as each modality can identify findings not detected by the other. For example, cortical can be used to determine the relative renal function of each renal moiety and may demonstrate other findings, such as a duplicated renal collecting system or cortical thinning due to hydronephrosis (► Fig. 10.3).
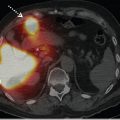
Occasionally, renal cortical SPECT will reveal a previously unappreciated renal anomaly, such as a horseshoe kidney or an ectopic kidney, and can be helpful for excluding an ectopic pelvic kidney in a patient with apparent renal agenesis (► Fig. 10.4).
A renal cyst or neoplasm will appear as a region of nonfunctional cortex on cortical SPECT, and, conversely, cortical renal SPECT can be used to demonstrate normally functioning renal cortex and the absence of a tumor. 29 , 30
Renal SPECT is used for functional evaluation of multicystic dysplasia of the kidney (MCDK), one of the most common cystic renal disorders in infants and children. MCDK typically appears as a conglomerate of noncommunicating renal cysts, with no or little apparent renal parenchyma. However, there is wide variation in the extent of cortical involvement, and cortical SPECT can be used to assess the amount of functional renal cortex in a dysplastic kidney.
Less commonly, cortical renal SPECT is used to assess residual and differential renal function in a patient with polycystic kidney disease.
In teenagers and young adults being evaluated for hypertension, cortical renal SPECT is used to exclude postinfection or posttraumatic renal scarring as a secondary cause of hypertension. 31 , 32


10.2.6 Correlative Studies
Correlation with renal ultrasound is always important for accurate interpretation of renal cortical SPECT. Renal ultrasound has become the standard modality for anatomical imaging of the kidney due to its widespread availability and absence of ionizing radiation. For example, identification of a duplex renal collecting system or renal cyst can be performed easily with ultrasound. However, ultrasound does not have perfect sensitivity for these abnormalities, and renal SPECT is complementary with renal ultrasound. 19 , 25 , 33 For example, abnormalities of renal structure, such as a duplex collecting system or horseshoe kidney, may not always be perceived on a renal ultrasound, but may be clearly present on renal SPECT images. Renal ultrasound may detect only about half of significant renal scarring detected by cortical renal scintigraphy. 19 , 25 , 33 A normal renal ultrasound may not exclude significant genitourinary pathology, 34 and ultrasound rarely detects scarring that is not demonstrated by renal SPECT.
In general, coregistration of renal SPECT with CT or MRI is not necessary, but correlation with available cross-sectional imaging, such as CT and MRI, can be helpful in complex cases.
10.3 Tumor Imaging with 123I-MIBG
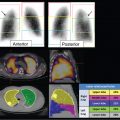
Neuroblastoma is the most common extracranial solid tumor in children. 35 Functional imaging with MIBG is an important part of tumor staging and patient management. 36 , 37 , 38 MIBG 39 is a catecholamine analogue that can be radiolabeled with either iodine-123 (123I) or iodine-131 (131I). It is a ligand of the norepinephrine transporter (NET), which facilitates reuptake of catecholamines, typically at autonomic nerve terminals. The NET is also expressed by nearly all neuroblastomas; therefore, radiolabeled MIBG can be used to localize and image these tumors. 40 In addition, 131I-labeled MIBG is used as an investigational agent for tumor-specific radiotherapy of neuroblastoma and other neuroendocrine tumors expressing the NET.
10.3.1 Technique
Radiopharmaceutical
For 123I-MIBG, the current harmonized guidelines 7 recommend an administered activity of 5.2 MBq/kg (0.14 mCi/kg) with a minimum of 37 MBq (1.0 mCi) and a maximum of 370 MBq (10.0 mCi).
Patient Preparation
Patent preparation is essential for MIBG scintigraphy to be technically adequate and clinically informative. 41 , 42 Patient preparation includes administering nonradioactive iodine to block uptake of unincorporated radioiodine by the thyroid gland and screening the patient’s medication list for drugs that may interfere with the study. Although thyroid gland damage is not a concern with 123I-MIBG, thyroid accumulation of 123I could obscure sites of disease in the neck. Some guidelines suggest large doses of nonradioactive iodine, usually in the form of potassium iodide. 42 Many medications have the potential to interfere with MIBG scintigraphy, and lists of interfering drugs are available for reference. 41 , 43 , 44 , 45 For most of these compounds, specific interference with MIBG scintigraphy has not been demonstrated, but they are included as they are similar to drugs that have been reported to interfere with MIBG scans. Some medications need special mention. Labetalol has well-defined interference and must be stopped before performing MIBG scintigraphy. 43 Over-the-counter cough and cold remedies may contain interfering compounds, and patients and families should be advised to avoid these medications. 42 Most current clinical guidelines recommend that potentially interfering drugs be discontinued for 2 to 3 days before MIBG scintigraphy. However, neuroleptic drugs, such as phenothiazines, administered in depot form may interfere if administered up to 1 month before the imaging study. Ideally, all potentially interfering drugs are discontinued before MIBG imaging, but it may not be clinically appropriate to discontinue some medications, such as antihypertensive medications, necessary to control the symptoms of catecholamine excess or neuropsychiatric drugs. The decision to discontinue a prescription medication in preparation for an MIBG scan must be made after consultation with the referring or treating clinician.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree