Hemochromatosis
Hemophilia
Hereditary Spherocytosis
Leukemia
Sickle Cell Anemia
Thalassemia
Vascular conditions that affect bone may manifest through alterations in the blood supply or changes in the skeletal architecture because of abnormal activation of the hematopoietic potential within bone marrow. Unfortunately, the slow metabolic rate of bone significantly delays visualization of quantitative, qualitative, and morphologic changes in the skeleton. The limited number of skeletal responses to hematologic alterations often impairs the physician’s ability to render a specific diagnosis based on plain films alone. In general, vascular conditions of the skeleton often have a chronic history of development and nonspecific radiographic findings. Consequently, all radiographic clues must be correlated with historic and laboratory findings. Typically the clinical findings suggest the correct diagnosis long before plain film radiographs are useful.
Avascular Necrosis
BACKGROUND
Avascular necrosis is the most common hematologic condition affecting the skeleton. Pathologically, this condition is simply bone death resulting from an inadequate blood supply. Avascular necrosis is more likely to develop when the blood supply to bone is tenuous or little collateral circulation is present. Many conditions and disorders can be responsible for the interruption of blood supply to a segment of bone. 10
The many conditions known to be responsible for avascular necrosis include trauma, alcoholism, infections, dysbaric trauma, marrow infiltration disorders, hypercoagulability states, autoimmune diseases, and idiopathic etiology. 3 These causes may be divided into three categories: (a) conditions resulting in external blood vessel compression near or within the bone, (b) disorders resulting in blood vessel occlusion because of thickening of the vessel wall, and (c) disorders resulting in blood vessel blockage from a thromboembolic process (Box 11-1). 11
BOX 11-1
Causes of Skeletal Ischemic Necrosis
External vessel compression
Trauma or surgery
Steroid administration
Idiopathic
Regional infection
Neuropathic joint
Gaucher disease
Hyperlipidemia
Vessel wall disorders
Systemic lupus erythematosus
Polyarteritis nodosa
Giant cell arteritis
Radiation therapy
Thromboembolic disorders
Alcoholism
Arteriosclerosis
Steroid administration
Thromboembolic syndrome
Diabetes
Trauma
Sickle cell disease
For conditions that result in external blood vessel compression, the mechanism is either marrow edema causing compression of the vessel in an enclosed region or excessive packing of the marrow through the deposition of abnormal tissue or material (e.g., fat in steroid administration or hyperlipidemia). 15,19,39Box 11-2 lists in descending order of frequency the common causes of ischemic necrosis in children and adults.
BOX 11-2
Most Frequent Causes of Ischemic Necrosis
Children
Idiopathic origin
Trauma
Infection
Adults
Drugs and other substances (steroids, alcohol, immuno-suppressants)
Trauma
Inflammatory arthritis
Metabolic conditions (diabetes, Cushing’s syndrome, pregnancy)
Bone ischemia can have several presentations. Infarction affecting a focal segment of the articular surface is termed osteochondritis dissecans in the growing skeleton. This defect may occur in adults and children, and it more frequently affects weight-bearing bones. The lateral aspect of the medial femoral condyle is the most common location of the infarction, followed by the talar dome. Trauma is believed to be the precipitating mechanism, although patients commonly report no history of trauma.
An infarction that affects the entire epiphysis of a skeletally immature long bone is termed epiphyseal ischemic necrosis. The proximal femur is by far the most common location for this event. 29 When the proximal femur is affected, the condition is called Legg-Calvé-Perthes disease (Box 11-3) in the child and Chandler disease in the adult. Early diagnosis of Legg-Calvé-Perthes disease is important in prevention of postischemic deformity and functional impairment. 24 Most cases of Legg-Calvé-Perthes disease have an idiopathic origin, although slipped femoral capital epiphysis, trauma, developmental dysplasia of the hip, and other pathologies also are associated with this process.
BOX 11-3
Legg-Calvé-Perthes Disease
Background
Idiopathic osteonecrosis of the proximal femoral capital epiphysis in children
Trauma, endocrine abnormality, and infection implicated as possible causes
More common among boys, whites, and those between 3 and 5 years of age
Imaging
Bilateral in approximately 15% of patients
Magnetic resonance imaging (MRI) and bone scans more sensitive to early disease than plain film radiographs
Bone scans cold during avascular phase and hot during revascularization phase
MRI scans revealing replacement of normal marrow by necrosis
Plain film findings of capsular distention, a small fragmented epiphysis, osteosclerosis (snowcap sign), subchondral collapse (crescent sign), radiolucent defect of the lateral margin of the involved epiphysis (Cage’s sign), curved radiodense cortical line at the base of the femoral neck representing the margin of the deformed femoral head (sagging rope sign), and growth deformity leading to a wide, short femoral neck with an enlarged (coxa magna), flattened (coxa plana) femoral head (mushroom) deformity
Clinical
Slowly evolving painless limp with limited abduction and internal rotation
Clinical outcome worse if weight-bearing area of bone involved
Osteoarthritis secondary to incongruent articular surfaces representing a significant complication
Treatment options of avoidance of full weight-bearing, bracing, and possible surgical realignment
Although ischemia of any segment of a bone is possible, certain locations are more likely to be affected because of their vascular supply (Table 11-1). An avascular etiology has been disproved for some epiphyseal conditions previously attributed to an avascular origin (Table 11-2).
| Location | Disorder |
|---|---|
| Metatarsal head | Freiberg disease |
| Tarsal navicular | Köhler bone disease |
| Talus | Diaz’s disease |
| Patella (secondary ossification center) | Sinding-Larsen-Johansson disease |
| Medial femoral condyle | Spontaneous osteonecrosis of the knee (SONK) |
| Femoral head (child) | Legg-Calvé-Perthes disease |
| Phalanges of hand | Thiemann’s disease |
| Metacarpal head | Mauclaire disease |
| Carpal lunate | Kienböck disease |
| Carpal scaphoid | Preiser disease |
| Capitellum of the humerus | Panner disease |
| Humeral head | Hass disease |
| Vertebral body | Kümmel disease |
| *See Chapter 8. | ||
| Condition | Etiology | Description |
|---|---|---|
| Blount’s disease (tibia vara) | Trauma | Local growth alteration of the medial portion of the proximal tibial epiphysis; infantile and adolescent presentation; depressed medial metaphysis of the tibia with an osseous overgrowth noted; shortening of involved leg; usually a tibia vara deformity |
| Osgood-Schlatter disease | Trauma | Altered appearance of the tibial tuberosity occurring mostly between the ages of 11 and 15 years; more common among boys and girls who participate in sports such as soccer and weight lifting; fragmentation and soft-tissue swelling of the tibial tuberosity evident on radiographs; pain and tenderness over the region; tibial tuberosity possibly fragmented as a normal variant and distinguishable from Osgood-Schlatter disease by lack of pain and soft-tissue swelling |
| Scheuermann’s disease* | Trauma | Posttraumatic defect of vertebral endplate maturation first seen during adolescence with three or more levels of wedged vertebrae, narrowed anterior disc space, multiple Schmorl’s nodes, and vertebral endplate irregularity; middle and lower thoracic spine are the usual locations; back pain is common; more severe kyphosis possibly necessitates bracing or, in a few cases, surgery to arrest or partially correct the resulting deformity |
| Sever’s phenomenon | Variation in ossification | Irregularity and fragmentation of the secondary ossification center of the calcaneus; generally considered a normal variant and unrelated to any heel pain in the adolescent |
| Sinding-Larsen-Johansson disease | Trauma | Fragmented appearance of the lower pole of the patella, most commonly occurring between 10 and 14 years of age; soft-tissue swelling and tenderness common and exacerbated by activity |
The histologic changes in bone necrosis are essentially the same, regardless of location. Pathologically, absence of a blood supply results in bone death within 48 hours; a predictable sequence of events then ensues: inflammatory reaction to the dead bone, neovascular infiltration into the dead segment of bone, resorption of dead bone and deposition of new bone, and remodeling of the resultant bone. 25 This process may take 1 to 8 years to complete.
IMAGING FINDINGS
Osteochondritis dissecans.
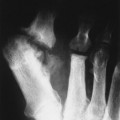
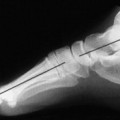
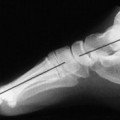
Osteochondritis dissecans (local subchondral infarction) generally first manifests radiographically, the plain film showing a subarticular defect accompanied by a free-floating fragment of bone that formerly filled the defect. The defect is characteristically smooth and regular, corresponding to the borders of the fragment (Fig. 11-1). The fragment may be displaced from the parent bone defect, or remain in site (in situ, meaning in itself). Marginal sclerosis is always present at the defect and blends into the subchondral bone peripherally (Fig. 11-2). These findings assist in differentiation of focal subchondral infarction from an acute, traumatic insult. Intraarticular swelling is often noted, but its severity varies considerably (Figs. 11-3 and 11-4).
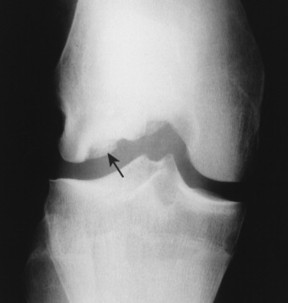 |
| FIG. 11-1 Osteochondritis dissecans. Focal osteochondral infarction of the lateral femoral condyle demonstrating smooth margins and a clear articular defect (arrow). |
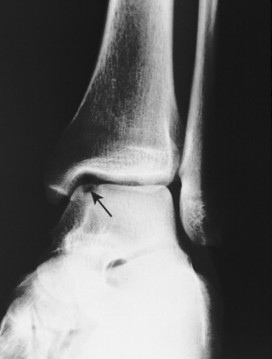 |
| FIG. 11-2 Osteochondritis dissecans of the ankle. Note the in situ osseous fragment at the superomedial aspect of the talar dome (arrow). (From Deltoff MN, Kogon PL: The portable skeletal x-ray library, St Louis, 1998, Mosby.) |
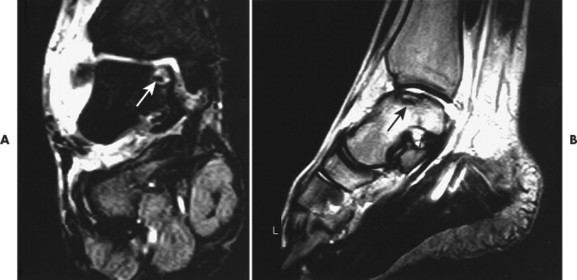 |
| FIG. 11-3 Osteochondritis dissecans appearing as a focal hyperintense defect on, A, coronal T2-weighted and, B, oblique T1-weighted sequences (arrows). Osteochondritis dissecans is a traumatic defect of the articular surface, common to the dome of the talus, particularly on the medial side. It is suggested clinically by persistent symptoms after an ankle strain. (Courtesy Robert Rowell, Davenport, IA.) |
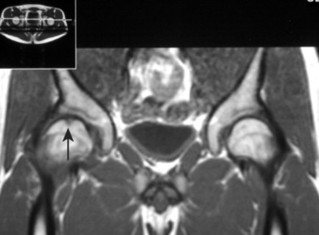 |
| FIG. 11-4 A small defect is noted at the top of the reading left femoral head, consistent with osteochondritis dissecans (arrow). |
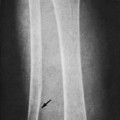
The plain film radiograph generally demonstrates no evidence of a medullary bone infarction in the acute phase. After resolution and revascularization, the infarction is seen radiographically as discontinuous, longitudinally oriented, and wavy or serpiginous calcific opacities that lie centrally in the medullary cavity (Figs. 11-5 and 11-6). The infarction does not result in periosteal reaction, alteration in the cortical thickness, or expansion of the bone. Differentiating a medullary bone infarction from a benign enchondroma of bone can be impossible on plain film images. However, definitive differentiation generally is unnecessary because both conditions are benign.
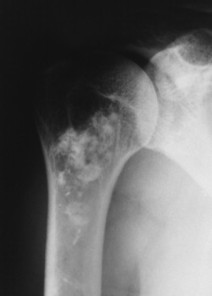 |
| FIG. 11-5 Irregular patchy sclerosis of a medullary bone infarction. |
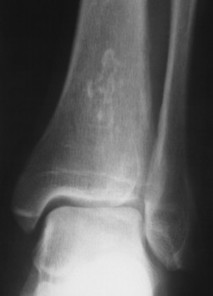 |
| FIG. 11-6 Subtle serpiginous calcifications in a medullary bone infarction. |
Epiphyseal necrosis
Avascular phase.
From both therapeutic and prognostic standpoints, epiphyseal necrosis is a significantly more important condition than medullary bone infarction. During the avascular phase of epiphyseal necrosis, plain film radiographic skeletal changes are absent (Fig. 11-7). 21 Intraarticular effusion may be visible, but the degree of effusion varies, making it an unreliable sign.
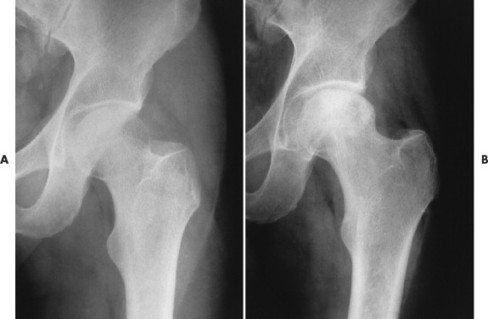 |
| FIG. 11-7 A, Normal plain films in a patient with a painful hip. B, Plain films 3 months later demonstrating increased radiodensity and a mottled appearance of the femoral head. These are typical signs of avascular necrosis of the femoral head. |
Changes in marrow signal can be identified with magnetic resonance imaging (MRI), which is considered the most sensitive imaging modality for the detection of early bone infarction. 5 MRI demonstrates loss in the epiphyseal marrow signal, particularly on T1-weighted images, even in the earliest phases of the disease (Fig. 11-8). 17,38
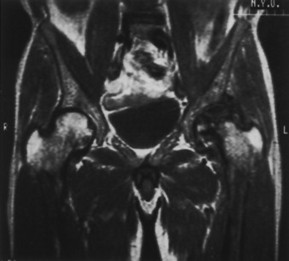 |
| FIG. 11-8 Avascular necrosis of the patient’s left (reading right) hip with severe secondary osteoarthritis on a coronal T1-weighted magnetic resonance imaging scan. (From Firooznia H et al: MRI and CT of the musculoskeletal system, St Louis, 1992, Mosby.) |
Early epiphyseal infarction also can be detected by radionuclide scintigraphy, which demonstrates focal photopenia affecting the avascular segment. Generally a zone of increased radionuclide uptake is present in the region surrounding the avascular segment, presumably resulting from hyperemia (Fig. 11-9). 8
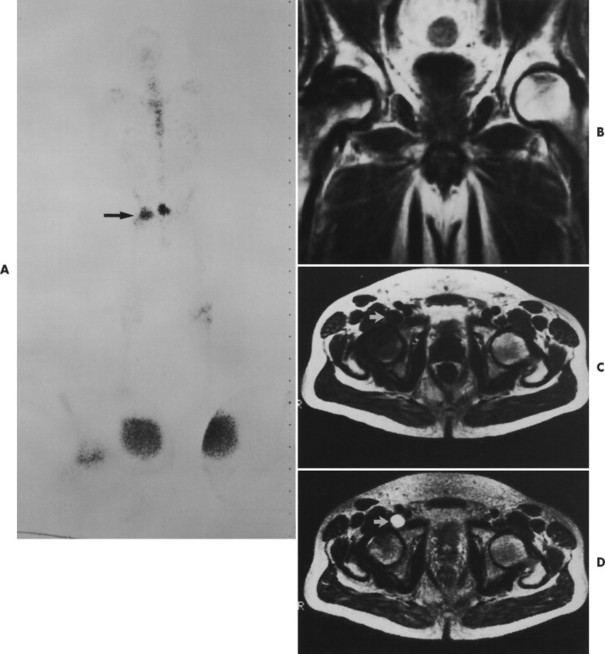 |
| FIG. 11-9 Images of a 52-year-old man who has right hip pain and is receiving steroids for systemic lupus erythematosus. A, A technetium 99mTc pyrophosphate bone scintigram shows increased uptake in the right hip (arrow) and a normal left hip. B, A coronal T1-weighted magnetic resonance image (MRI) shows obvious avascular necrosis on the right and subtle characteristic avascular necrosis changes on the left. Core biopsies confirmed bilateral avascular necrosis. C, Axial T1-weighted MRI. D, Axial T2-weighted MRI. Fluid is shown (arrows) in the right iliopsoas bursa. Iliopsoas bursitis may accompany avascular necrosis and cause a variety of symptoms. (From Firooznia H et al: MRI and CT of the musculoskeletal system, St Louis, 1992, Mosby.) |
Inflammatory phase.
As the avascular phase gives way to the inflammatory response in epiphyseal necrosis, plain films may demonstrate periarticular osteopenia affecting all but the involved segment of bone. This may create the plain film appearance of sclerosis of the avascular segment, but it is not an infallible radiographic finding (Fig. 11-10). The inflammatory phase may persist for weeks.
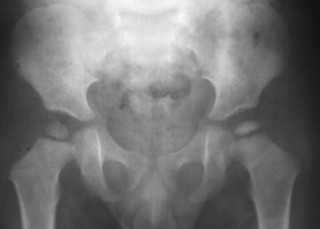 |
| FIG. 11-10 Anteroposterior projection of the pelvis demonstrating a small radiodense epiphysis on the reading right. These changes indicate ischemic necrosis of the epiphysis (Legg-Calvé-Perthes disease) in this child. |
During this time the dead bone begins to soften and may collapse under the stresses of use. With this collapse the involved articular surface becomes deformed (Figs. 11-11 and 11-12). Subchondral fractures allowing joint fluid to intrude into the subchondral ischemic bone may produce the “crescent” sign on plain films. This sign appears as a lucent defect in the subchondral bone that parallels the subchondral cortical surface (Fig. 11-13). The crescent sign is considered the most reliable early plain film sign of epiphyseal infarction. 21 Larger regions of bone collapse may develop, appearing as semilunar radiolucent defects extending from the articular cortex. The defects have been likened to configuration of a bite mark, known as bite sign. A variation of this is designated by the Gage’s sign, a radiolucent defect of bone (often shaped as a V) appearing at the lateral margin of the involved epiphysis in patients with Legg-Calvé-Perthes disease.
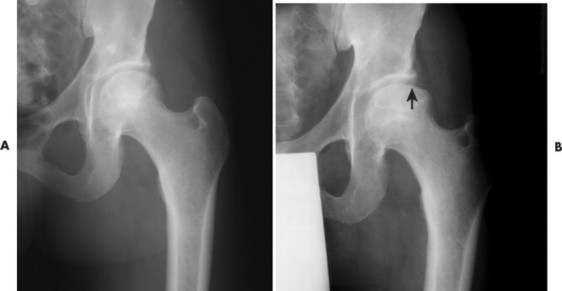 |
| FIG. 11-11 Avascular necrosis. A, The first radiograph of this 32-year-old woman appears normal. B, However, 1 year later, clear signs of necrosis are evident by the increased radiodensity of the femoral head and the flattened appearance of the femoral head’s articular cortex (arrow). |
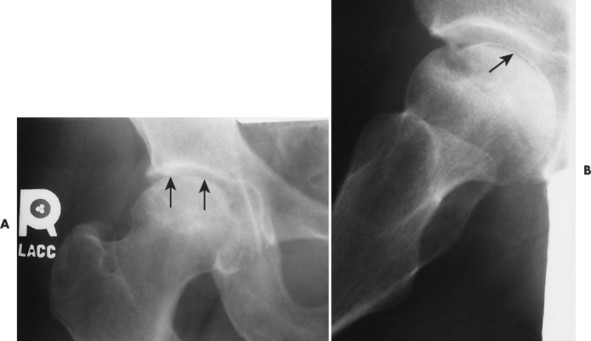 |
| FIG. 11-12 A and B, Ischemic necrosis indicated by a radiolucent crescent sign is faintly visible on the anteroposterior view of the hip but is obvious on the frog-leg lateral view (arrows). Mottled bone sclerosis of the femoral head is also noted. |
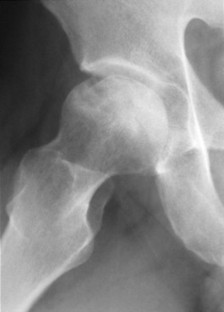 |
| FIG. 11-13 Articular collapse in a patient with adult-onset ischemic necrosis of the femoral head. |
Revascularization phase.
As revascularization continues, the dead bone is resorbed while new bone matrix is being deposited to replace it. On radiographs this process is seen as apparent fragmentation of the epiphysis. In addition, sclerosis begins to appear at the margin of the necrotic segment, producing a mottled appearance (FIG. 11-14FIG. 11-15FIG. 11-16 and FIG. 11-17). Articular collapse is common (Fig. 11-18). The fragmentation gradually recedes as the new epiphyseal bone ossifies.
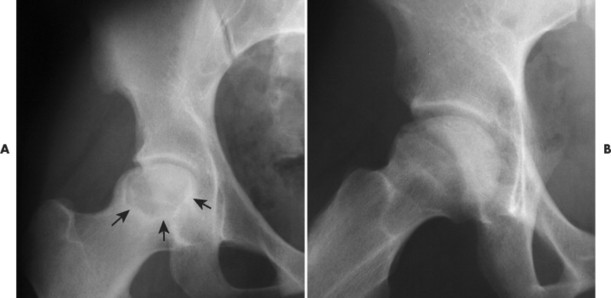 |
| FIG. 11-14 A and B, The femoral head appears mottled, with a semicircular defect extending into the femoral head from the superior cortical margin (“bite sign”) (arrows). |
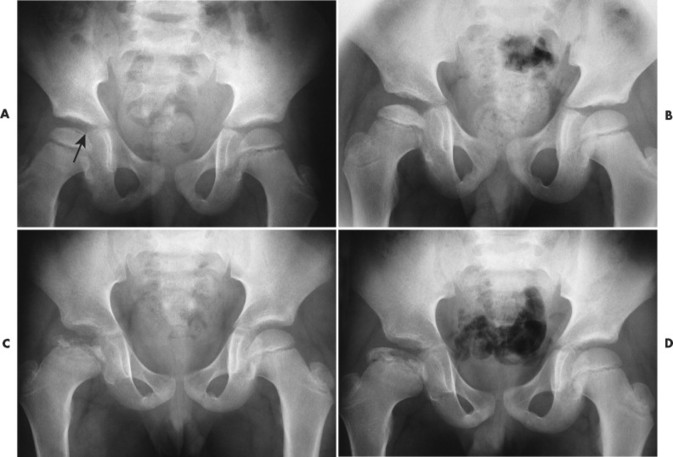 |
| FIG. 11-15 Legg-Calvé-Perthes disease. A, Early changes of Legg-Calvé-Perthes disease. Note the lucent crescent sign (arrow) at the superior aspect of the femoral epiphyseal surface. B through D,
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|