11 Hydrocephalus
11.1 Introduction
Hydrocephalus is the pathologic accumulation of excess cerebrospinal fluid (CSF) within the head, typically within the ventricular system and typically with increased intraventricular pressure. It can be seen both on a congenital and an acquired basis, and it is a condition frequently encountered in pediatric neuroradiology. Although the most basic understanding of hydrocephalus is easy to grasp, the frequency and complexity of this disease process make it helpful to understand more about the pathophysiologic mechanisms responsible for it as well as treatment options for it.
11.2 Basic Model for Hydrocephalus
The most basic model for understanding hydrocephalus, which admittedly is now known to be a gross oversimplification, remains a reasonable starting point in describing thes condition. Cerebrospinal fluid (CSF) is predominantly formed by the choroid plexus in the atria of both lateral ventricles of the brain. The CSF then travels through the foramina of Monro into the third ventricle (Fig. 11.1), with the fluid propelled in a pulsatile manner by displacement resulting from arterial expansion during systole. From the third ventricle, CSF traverses the sylvian aqueduct (between the mesencephalic tegmentum and quadrigeminal plate). From the fourth ventricle, outflow of CSF occurs inferiorly into the cisterna magna through the foramen of Magendie, and laterally into the lateral medullary cisterns through the foramina of Luschka. Cerebrospinal fluid is eventually resorbed by the dura and arachnoid granulations, and enters the blood.
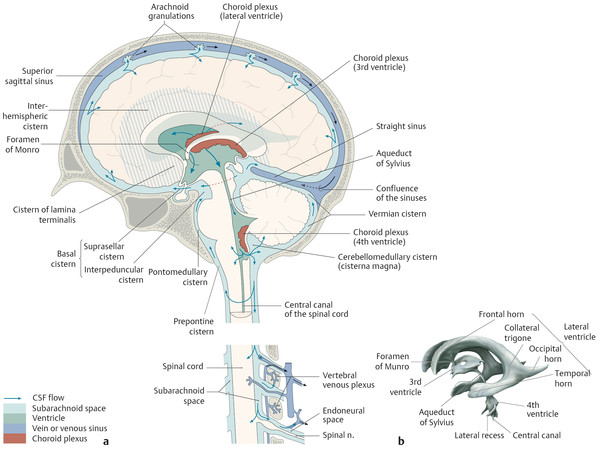
It is estimated that in adults, up to 500 mL of CSF is produced per day, but the volume of CSF at any given time is approximately 150 mL, indicating a multifold turnover of the entire volume of CSF every day. Thus, there is a balance between the volume of CSF produced, CSF transit, and the ability to resorb CSF. Alterations in any of these factors can result in hydrocephalus.
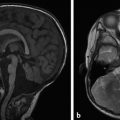
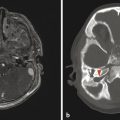
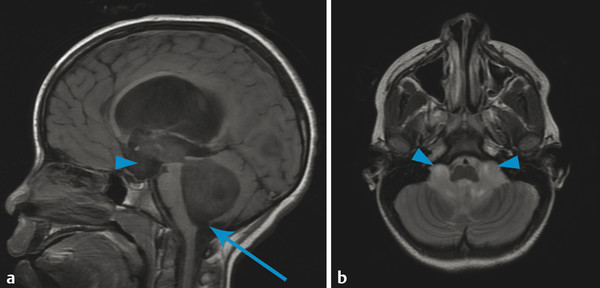
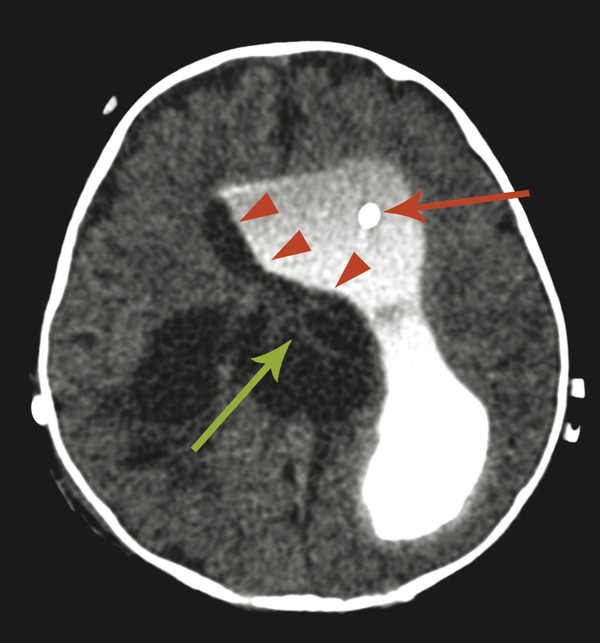
The most commonly encountered pattern of hydrocephalus relates to obstruction of the aqueduct of Sylvius (aqueductal stenosis or obstruction) (Fig. 11.2). The sylvian aqueduct can be narrowed or obstructed by blood products after intraventricular hemorrhage or by inflammatory debris in the setting of meningitis, or it can be extrinsically compressed by a mass (such as a pineal tumor or tectal glioma). Aqueductal stenosis can be congenital with an X-linked form of the disorder. It is also an associated feature in many cases of rhombencephalosynapsis, an abnormality in hindbrain cleavage that results in incomplete lateral migration of the inferior colliculi (mesencephalosynapsis) and resulting underdevelopment of the inferior aspect of the sylvian aqueduct.
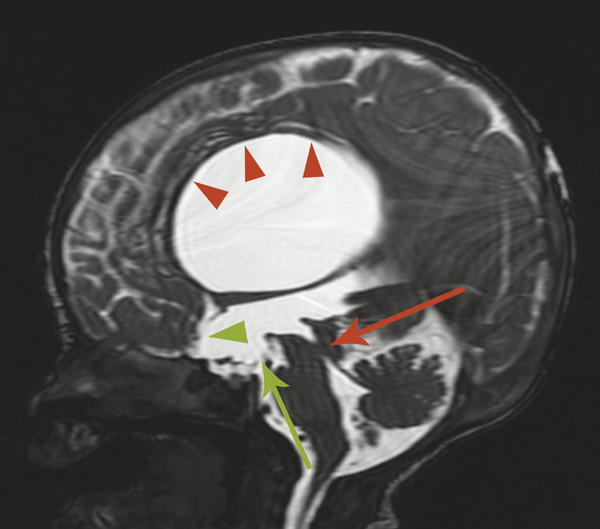
Stenosis or obstruction of the sylvian aqueduct will result in excessive accumulation of CSF within the lateral and third ventricles, a pattern known as triventricular hydrocephalus. At varying degrees of severity of stenosis, there will be fullness of the frontal and temporal horns of the lateral ventricles, anterior bowing of the lamina terminalis, inferior bowing of the floor of the third ventricle, and splaying of the chiasmatic and infundibular recesses of the third ventricle. The fourth ventricle is not dilated in this condition. Historically, stenosis or obstruction of the sylvian aqueduct has been referred to as obstructive hydrocephalus.
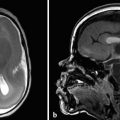
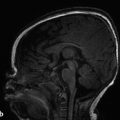
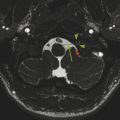
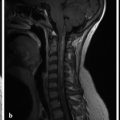
If the fourth ventricle is also dilated, in a pattern referred to as tetraventricular hydrocephalus, (Fig. 11.3), the assumption is that the sylvian aqueduct of is patent. This has historically been ascribed to the overproduction and/or underabsorption of CSF, and it is referred to as communicating hydrocephalus; however, this term is often inaccurately applied and misleading. Many patients with a tetraventricular hydrocephalus actually do have an obstruction, with arachnoid webs or membranes obstructing the outflow of CSF from the fourth ventricle (Fig. 11.3). A persistent Blake’s-pouch cyst without fenestration is a consideration for a fourth ventricular outflow obstruction. It is known that patients with meningeal irritation/inflammation from meningitis can have impaired CSF resorption resulting in tetraventricular ventriculomegaly, and they may possibly also have prominence of the extra-axial space. This prominence is more common in the neonate because of the ability of the neonatal skull to enlarge (Fig. 11.4).
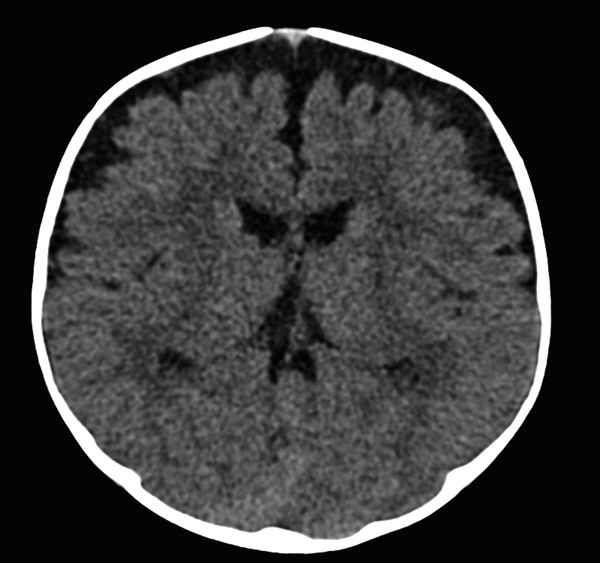
Hydrocephalus with isolated enlargement of the subarachnoid space without signs of ventricular enlargement has been referred to as external hydrocephalus. This term is confusing and often mistakenly applied; most patients suspected of having this condition actually have normal pressures and instead have a condition known as benign enlargement of the subarachnoid spaces of infancy (BESSI) (further described below) (Fig. 11.5).
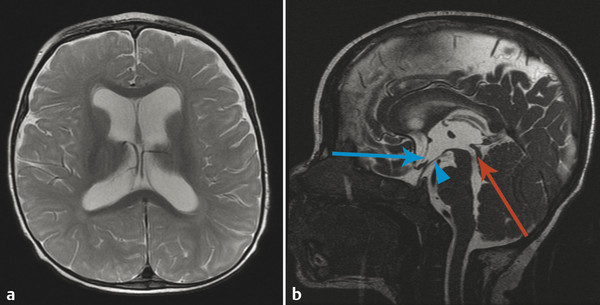
Similarly, it is possible for a patient to have enlarged ventricles (ventriculomegaly) without having any abnormal CSF pressures or imbalance in CSF production/transit/resorption. The most common setting in which this is encountered is that of juxtaventricular parenchymal volume loss, which results in ex-vacuo enlargement of the ventricular system (Fig. 11.6). This is a situation in which ventriculomegaly can exist without hydrocephalus, a condition that would not benefit from procedures for diverting CSF flow, such as the placement of a ventriculoperitoneal (VP) shunt. However, a common cause of parenchymal volume loss in children is prior germinal matrix hemorrhage and/or white-matter disease of prematurity, which themselves can result in hydrocephalus. Therefore, a patient can have hydrocephalus superimposed upon ex-vacuo ventriculomegaly. Differentiation between the conditions can be challenging; however, it is important to be aware that because pure ex-vacuo ventriculomegaly is associated with normal intraventricular pressures, there will be no evidence of abnormal splaying of the third ventricular recesses and the sylvian aqueduct should be patent.
An additional and more complicated type of hydrocephalus is complex post hemorrhagic hydrocephalus, which is commonly encountered in premature infants. In addition to obstruction of the sylvian aqueduct, this condition may include loculated membranes and adhesions within the lateral ventricles, possibly requiring either cyst fenestration or the placement of separate shunt catheters within each lateral ventricle (Fig. 11.7).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree