11 Pediatric Intestinal Ultrasonography
Ultrasonography (US) is the imaging modality of choice for the initial evaluation of intestinal diseases in children for many reasons:
It is relatively inexpensive.
It is patient-friendly.
It lacks radiation and motion artifacts.
The small size of the child compensates for the limited penetration of sound waves.
The small size of the child facilitates the use of high-frequency transducers.
US involves direct contact with the patient, offering a unique opportunity to ask specific questions and perform additional physical examination, emphasizing the role of the radiologist as a clinician.
Flow studies are possible with the Doppler mode.
Real-time imaging allows visualization of movements (e.g., peristalsis).
Actually, US has become the most important imaging technique in children and can be considered the workhorse of pediatric radiology.
Initially, US of the stomach and intestines was not popular for obvious reasons: bowel gas has the annoying characteristic of reflecting all sound waves or creating artifacts because of its abnormally low acoustic impedance. However, increased knowledge, improved technique (e.g., graded compression), improved hardware (high-frequency transducers), and improved software (adaptive imaging, compound imaging) have changed this concept. Nowadays, it is impossible to imagine US without intestinal US, especially in pediatrics!
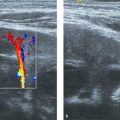
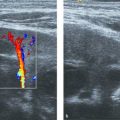
The hallmark of intestinal ultrasonography is the “gut signature.” This is the characteristic appearance of the layers of the gut ( Table 11.1 ; Fig. 11.1 ). The wall of the intestine is considered stratified when the submucosal echogenicity is present and the mucosa, submucosa, and muscularis propria are separately visible. Nonstratification means indistinctness of the mucosa and submucosa or of all layers.
Layer | Echogenicity |
Mucosal surface | Hyperechoic |
Mucosa | Hypoechoic |
Submucosa | Hyperechoic |
Muscularis propria | Hypoechoic |
Serosal surface | Hyperechoic |
Here are some practical tips for a successful intestinal ultrasonographic examination:
Have patience. Infants have limited sympathy for the workload of a pediatric radiologist.
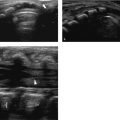
Use graded compression whenever necessary, always realizing that children are not as delicate as porcelain ( Fig. 11.2 ; Video 11.2). If they can withstand vaginal delivery, they can tolerate serious graded compression.

Fig. 11.2 a, b Juvenile polyp in the descending colon. Without compression, a normal descending colon is seen filled with gas (a). Mild compression reveals a juvenile polyp (between arrows, b).
Use warm ultrasound gel. It is almost unimaginable how a peacefully sleeping infant can be turned into a raging beast solely by administering cold gel to its belly.
Take advantage of domestic sedation whenever possible. A pacifier with a few drops of syrup will do the trick. The infant will stop crying and relax the abdomen. We have syrup in every US room. Some parents object to the administration of nonsterile, cariogenic, obesity-inducing drugs that inevitably will lead to overindulgence. For these reluctant parents, sterile sucrose (24%) is available in the neonatal intensive care unit in single-patient twist cap vials for the same purpose and the same results at a greater expense ( Fig. 11.3 ).

Fig. 11.3 a, b Syrup (not diluted!) and pacifier (a) and a single-patient vial of 24% sucrose (b) for annoying infants.
Start the examination with a structure that always will be recognized—the descending colon. That will give you confidence.
A small amount of peritoneal fluid at the base of the cecum and behind the bladder is physiologic in children.
Try to hold the transducer still for a while, and try to appreciate the bowel movements (or absence of peristalsis) and the passage of gas bubbles through vessels or a fistula. Gentle compression of the transducer at a fixed position will reveal fluctuation of unexpected pus.
Make liberal use of color Doppler US. It may reveal the solid nature of hypoechoic infiltrates or lymph nodes (simulating fluid) and the avascularity of an apparently normal bowel loop.
Observe at least the following: length of the affected segment, wall thickness, degree of stratification, vascularity, presence of peritoneal fluid (and its clarity), and peristalsis.
Be aware of hyperechoic islands of mesentery or omentum with hypoechoic surroundings ( Fig. 11.4 ; Video 11.4). In our experience, they are virtually pathognomonic for infiltrative pathology (inflammatory or neoplastic).

Fig. 11.4 a, b Isolated hyperechoic islands of mesenteric or omental fat (arrowheads). a Patient with mesenteric Burkitt lymphoma and infiltrative invasion of the mesentery, best seen on the video. b Patient with peritonitis caused by necrotic and perforated intussusception. Note the slight dilatation of the appendix (open arrowhead). Only the base of the appendix was involved in the intussusception (see Video 11.4).
Always be aware of this: “An uncommon presentation of a common disease is more common than a common presentation of an uncommon disease” ( Fig. 11.48 and Fig. 11.49 ).
This chapter provides an overview of the diagnostic potential of pediatric gastrointestinal US.
11.1 Esophagus
Endoscopic US is a useful technique to evaluate the esophageal wall and its adjacent mediastinum in a variety of congenital and acquired diseases. However, it is an invasive method that requires sedation or anesthesia and will not be discussed in this chapter on conventional US.
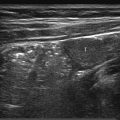
The cervical esophagus can be visualized by conventional US in all children as an oval structure between the trachea and vertebral column. It can be seen to a better advantage by using the thyroid gland as an acoustic window and rotating the head 45 degrees to the opposite site ( Fig. 11.1a). The esophageal wall can be recognized by its gut signature with five clearly defined layers ( Table 11.1 ). The mean wall thickness is 2.8 mm (range, 2.2–3.8 mm) at all ages.
In patients with esophageal atresia without a tracheoesophageal fistula, the abdomen can be examined with US to reveal additional abnormalities (Fig. 11.5). More on esophageal atresia is presented in Chapter 6 (Section 6.2.3 Esophagus).

11.2 Gastroesophageal Junction
The gastroesophageal junction ( Fig. 11.6 ) can be visualized with US in 87 to 95% of children with suspected gastroesophageal reflux disease (GERD), and reflux of the gastric contents into the esophagus can be demonstrated. A threshold of three reflux periods within 10 minutes has a sensitivity of 82% and a specificity of 85% for GERD. Farina et al increased the sensitivity to 98% by using color Doppler US. However, in premature infants, the results differ; the sensitivity is 38% and the specificity is 100% when compared with 24-hour PH-metry.

US studies have also shown that there is a positive correlation between GER and the length of the abdominal esophagus, protrusion of the gastric mucosa, and an increased gastroesophageal angle (angle of His). Therefore, US can be the initial imaging technique in patients suspected of having GERD. Moreover, US can evaluate the postoperative situation after a fundoplication and gastric emptying, another important contributing factor to the pathogenesis of GERD.
11.3 Stomach
US can be used to evaluate gastric emptying in patients with vomiting. Both dynamic and anatomical abnormalities can be depicted. A food-filled stomach should not be mistaken for a tumor ( Fig. 11.7 ).

Gastric duplication cysts: The stomach is the second most common location of duplication cysts ( Fig. 11.8 ). A detailed discussion of duplication cysts is found in the section of this chapter on the small bowel.

Gastric volvulus (mesenteroaxial and organoaxial) in children is rare. In the organoaxial type (more common), the rotation is along the longitudinal axis of the stomach. In the mesenteroaxial type, the stomach twists along the axis of its mesentery and flips into an inverted position ( Fig. 11.9 ). Volvulus is facilitated by poor fixation by the gastric ligaments and gastroperitoneal attachments. It is associated with diaphragmatic herniation, eventration, asplenia/polysplenia syndrome, and hypermobility of the spleen. The typical presentation is vomiting with epigastric distention. US signs are gastric dilatation caused by gastric outlet obstruction and an abnormal position of the antrum in the mesenteroaxial type, ventral and superior to the fundus ( Fig. 11.9 ; Video 11.9).

Gastritis and peptic ulcer disease are uncommon in children. US shows a small lumen and stratified wall thickening with hyperemia. In severe cases (i.e., accompanied by deep ulcer disease), the stratification may become less distinct. When present, the ulcer is difficult to visualize. Eosinophilic gastritis is a rare disorder often involving the antropyloric region that may even cause gastric outlet obstruction, simulating hypertrophic pyloric stenosis. Stomach wall stratification is usually preserved. The detection of multifocal small-bowel involvement is highly suggestive of the diagnosis.
Foveolar hyperplasia consists of a polypoid thickening of the mucosal layer. It may be seen after long-standing prostaglandin therapy ( Fig. 11.10 ), hypertrophic gastropathy, or cow’s milk allergy, or it may be idiopathic ( Fig. 11.11 ; Video 11.11). It may simulate pyloric hypertrophy, but on closer US examination, the obstruction will appear to be caused by thickened mucosa instead of thickened muscle ( Fig. 11.11c, d).


Other rare causes of gastric wall thickening and gastric outlet obstruction include pyloric spasm, food allergy, chronic granulomatous disease, hyperlipidemia, chemotherapy ( Fig. 11.12 ; Video 11.12), duplication cysts ( Fig. 11.8 ), ectopic pancreas, benign and malignant tumors, and bezoars.

Hypertrophic pyloric stenosis: Infantile hypertrophic pyloric stenosis is of unknown etiology. It affects young infants ages 2 to 8 weeks, in whom the antropyloric portion of the stomach becomes abnormally thickened and obstructs gastric emptying. The male-to-female ratio is approximately 4:1.
Typically, infants with infantile hypertrophic pyloric stenosis are clinically normal at birth; during the first few weeks of life, they develop nonbilious, “projectile” vomiting that leads to weight loss, dehydration, and hypochloremic alkalosis, and eventually to death.
Surgical treatment is curative. The clinical diagnosis relies on palpation of the thickened pylorus. Abdominal palpation is accurate but not always successful, depending on factors such as the experience of the examiner, the presence of gastric distention, and the cooperation of the infant.
In virtually all patients, US is very accurate in facilitating the diagnosis. US, therefore, plays a key role in the initial care of these infants. It is important that the radiologist understands the anatomical changes of the pyloric channel in affected infants as reflected by US, which shows pyloric muscle hypertrophy to a variable degree during the examination. Also, a certain amount of thickening of the mucosa is present ( Fig. 11.13 ; Video 11.13a, b). A muscle thickness of 3 mm or more throughout the examination is considered to be diagnostic of infantile hypertrophic pyloric stenosis, although some authors state that the overall morphology and dynamic impression during the examination are as important, including the length of the pyloric canal, relaxation, and peristalsis. The examination can be performed in a very short time with an accuracy approaching 100%. Inexperienced sonographers are encouraged to introduce a nasogastric tube (many children already have one) to remove the air and introduce 20 mL of clear fluid (e.g., saline) and to turn the child in an oblique right-sided position (to pour the water into the antrum). This will show the anatomy to the best advantage. And always remember that the pylorus lies next to the gallbladder.

11.4 Small Bowel
Conventional radiography and US are the initial imaging modalities in children with abdominal pain or obstruction. The most important additional value of US over conventional abdominal radiography in these children is the capability to visualize peristalsis, vascularity, bowel-wall characteristics, dilatation of fluid-filled loops, and extraintestinal abnormalities (e.g., ascites and other fluids). The jejunum and ileum can be distinguished from the colon based on their anatomical location, caliber, contents, folds, and peristalsis.
Anatomical location: The colon has a peripheral location in which the ascending colon and descending colon lie dorsally in both flanks and the transverse colon is located ventrally in the upper abdomen. The sigmoid colon traverses the left psoas muscle and courses into the pelvis. In contrast, the small bowel has a more central position.
Caliber: The diameter of the small bowel is small and the diameter of the large bowel is relatively large (explaining the nomenclature).
Contents: The small bowel is either empty or filled with liquid contents and little air, whereas the colon is generally filled with gas-filled, bulky stool.
Folds: The folds in the jejunum are more numerous, longer, thinner, and closer together than the ileal folds. In the terminal ileum, the mucosa may be thickened by hyperplasia of lymphoid tissue. The colon is recognized by its haustra. Infants have few or no haustra.
Peristalsis: The small bowel is continuously moving because of peristaltic waves, whereas the colon shows sparse movement.
Baud proposed a systematic US approach for differentiating small-bowel disease, based on wall thickening.
Determine wall thickening: normal, up to 3 mm; mild thickening, 3 to 6 mm; moderate thickening, 6 to 9 mm; severe thickening, more than 9 mm.
Determine location (proximal or distal) and extent (focal, 5 cm; segmental, 6–40 cm; diffuse, more than 40 cm)
Determine stratification. Bowel wall is stratified when the hyperechogenicity of the submucosa is preserved and the mucosa, submucosa, and muscularis propria are visible as separate layers. Nonstratification means the absence of a distinction between the mucosa and submucosa or between all three layers ( Fig. 11.1 and Fig. 11.18 ).
Determine the valvular fold pattern; normal, thickened, thumbprinting, or hyperplastic valvular folds.
In general, thickened small-bowel loops show decreased peristalsis and contain little air, and they are therefore easily visualized and measured. At least three patterns can be distinguished:
Stratified thickening of the small bowel is found in infectious ileitis, advanced appendicitis, early Crohn disease, and graft-versus-host disease.
Nonstratified thickening of the small bowel is found in Henoch–Schönlein purpura, advanced Crohn disease, tuberculous ileitis, protein-losing enteropathy, hereditary angioedema, ischemia, celiac disease, Burkitt lymphoma, Kawasaki disease, and viral enteritis.
Nonstratified thickening with hyperplastic valvular folds is found in viral (and sometimes bacterial) lymphoid hyperplasia and Yersinia ileitis.
Malrotation and midgut volvulus : The portion of the intestine that is supplied by the superior mesenteric artery is referred to as the midgut, extending from the midportion of the second part of the duodenum to the distal third of the transverse colon. The term malrotation is widely used to describe the spectrum of developmental anomalies that are associated with abnormal position and fixation of the midgut. Patients with malrotation have a mesenteric root with a small fixation to the retroperitoneum. This situation predisposes to midgut volvulus, which is a life-threatening event that can result in necrosis of the entire small bowel.
No single technique is 100% accurate in diagnosing malrotation, and one should weigh the balance of evidence from all examinations to determine the correct diagnosis. Most institutions prefer the use of upper gastrointestinal series in cases of suspected malrotation, but technical difficulties and problems of interpretation may result in inconclusive results. These problems are also encountered with the barium enema.
US is gaining importance for the initial work-up of patients with suspected malrotation/volvulus. US features of malrotation are an abnormal relation between the superior mesenteric artery (SMA) and superior mesenteric vein (SMV), wide proximal duodenum, absence of the D3 (horizontal) segment of the duodenum, and abnormal position of the cecum and ascending colon. The normal anatomical position of the SMV is to the right of the SMA in the transverse plane ( Fig. 11.14 ). This is easily visualized with US, even in patients with overlying air-filled bowel loops, by using graded compression or sedation (see introduction). Greater deviation from this normal anatomical relation increases the sensitivity for malrotation ( Fig. 11.15 and Fig. 11.16 ).



In a series of 211 children suspected of having malrotation/volvulus, the sensitivity and specificity of an anterior–posterior orientation of SMV and SMA for malrotation were 19% and 81%, and the positive predictive value (PPV) and negative predictive value (NPV) were 10% and 90%. In contrast, the sensitivity and specificity of an inverted SMV and SMA for malrotation were 71% and 89%, and the PPV and NPV were 42% and 97%.
The normal anatomical position of the D3 (horizontal) segment of the duodenum is between the aorta and the SMA. Moving the transducer in the transverse plane in a craniocaudal direction, one will first encounter the root of the SMA, second the traversing left renal vein, and finally the duodenum ( Fig. 11.14 ; Video 11.14). According to Yousefzadeh, a normal position of the D3 segment virtually excludes the presence of malrotation.
In midgut volvulus, the midgut is twisted around its vascular pedicle, facilitated by the small mesenteric base in patients with malrotation. US in the transverse plane shows the whirlpool sign ( Fig. 11.17 ). In this situation, the SMV twists around the SMA when the transducer is moved in a craniocaudal or caudocranial direction (Video 11.17). The sensitivity and specificity of the whirlpool sign for volvulus were 45% and 99%, and the PPV and NPV were 71% and 97%.

Crohn disease is a chronic inflammatory bowel disease characterized by a granulomatous component, transmural inflammation, a tendency to affect the surrounding tissues, and the formation of fistulas and sinus tracts. The exact cause is still unknown. Crohn disease can occur after 5 years of age, but most often adolescents and young adults are affected. The initial course is insidious, with recurrent diarrhea, abdominal pain, and weight loss. Also, atypical features as growth failure, malnutrition, anorexia nervosa, amenorrhea, arthritis, and cutaneous and ocular manifestations may be present. Levels of calprotectin in the stools are increased. Calprotectin is a neutrophil protein that is released from activated leukocytes. Although the calprotectin level is a relatively new test, it is regularly used as an indicator of inflammatory bowel disease. Crohn disease can affect the digestive tract from mouth to anus but has a predilection for the terminal ileum. Initially, the inflammation starts as aphthoid ulcers in the mucosa and progresses to a transmural inflammation that eventually also involves the surrounding fat. Finally, the inflammation may proceed to other surrounding structures, such as the skin, bowel loops, and urinary, bladder forming sinus tracts, fistulas, and even abscesses.
On US, the bowel-wall stratification is initially preserved ( Fig. 11.18a). In advanced disease, the stratification is lost, and the affected bowel loop is surrounded by thickened, inflamed, echogenic fat, more or less isolated from the other bowel loops ( Fig. 11.18b, Fig. 11.19 , Fig. 11.20 ). Close attention should be paid to small hypoechoic spurs that extend into the echogenic fat. They probably represent insidious sinus tract formation and predict future fistula or abscess formation ( Fig. 11.21 , Fig. 11.22 , Fig. 11.23 ; Video 11.21).






US is of great value in the diagnosis and follow-up of children with inflammatory bowel disease. It has a sensitivity for inflammatory bowel disease of 75 to 88% and a specificity of 82 to 93%. Faure even reports a sensitivity of 100% for terminal ileitis in Crohn disease.
Intestinal polyps in the small bowel are usually hamartomatous polyps in patients with Peutz–Jeghers syndrome ( Fig. 11.24 ; Video 11.24). These polyps occur more often in the jejunum than in the ileum. US is able to detect moderate and large polyps and therefore may serve as a screening method for the detection of uncomplicated polyps. However, one should take into account the high specificity but relatively low sensitivity. US is valuable in diagnosing complications associated with small-bowel polyps (e.g., intussusceptions). One should be aware that specific particles within the stools may mimic polyps (e.g., undigested food, foreign bodies; Fig. 11.25 ; Video 11.25).


Henoch–Schönlein purpura is a systemic vasculitis of unknown cause involving the small vessels of the skin, gut, and kidneys in children between approximately 3 and 8 years of age. Patients develop a typical skin rash and intestinal problems such as nausea, vomiting, abdominal pain, melena, and bloody stools. Arthralgia, proteinuria, and hematuria are additional findings. Abdominal symptoms are present in two-thirds of the patients and can even mimic acute abdomen. The intestinal symptoms may precede the skin rash, and therefore the radiologist may be the first to suggest the diagnosis.
The small bowel is affected, including the duodenum ( Fig. 11.26 a). Involvement is frequently multifocal with skipped lesions. US findings include symmetric thickening of the bowel wall up to 11 mm, loss of stratification, intramural hematoma, enlargement and smoothening of folds, hypoperistalsis, hyperemia, lymphadenopathy, and often some anechoic peritoneal fluid ( Fig. 11.26 ; Video 11.26a, b). These US findings can subside within several days, and exacerbations and remissions may occur. The sensitivity, specificity, PPV, and NPV of US for gastrointestinal involvement with Henoch–Schönlein purpura are 83%, 100%, 100%, and 54%, respectively.

Complications include pathologic small-bowel intussusception, bleeding, and rarely necrosis and perforation, pancreatitis, cholecystitis, and appendicitis. Henoch–Schönlein purpura is a self-limiting disease, although persisting proteinuria due to nephritis is a well-known complication ( Fig. 11.26d).
Note the abnormally decreased echogenicity of the submucosa (arrowheads), thought to represent fluid accumulation within the submucosa caused by the vasculitis, and the peritoneal fluid (arrow, Fig. 11.26c). The hypoperistalsis and hyperemia are best appreciated on the videos.
Increased echogenicity of the right kidney is due to nephritis ( Fig. 11.26d). A small rim of peritoneal fluid is seen between the liver and the kidney. Numerous enlarged mesenteric lymph nodes are present ( Fig. 11.26e).
Ascaris lumbricoides is the most common helminthic infestation wordwide, affecting mainly children and young adults. Many children are not symptomatic or have minor, nonspecific abdominal complaints. The US images are quite characteristic, depending on the image plane; in the longitudinal plane, the worms appear as parallel echogenic lines, whereas in transverse images, they appear as round dots inside the lumen ( Fig. 11.27 ). The administration of fluid before the examination improves visualization of the worms. Differentiation from intraluminal lines may be difficult, and the movement of the worms can aid in the differentiation. Tubes and lines do not move by themselves. Ascaris residing in the small intestines may migrate into the biliary system, pancreatic duct, and appendix, causing symptoms of biliary stones, pancreatitis, or appendicitis.

Meckel diverticulum most commonly presents as painless rectal bleeding due to ulceration caused by ectopic gastric mucosa. These patients are usually adequately diagnosed and managed following a radionuclide scan.
In fewer than 50% of children presenting with Meckel diverticulum, the clinical findings will be more complex, with a combination of abdominal pain, vomiting, and occasionally rectal bleeding. In children with acute pain, the diagnosis is often difficult and nonspecific. US can be used successfully to document the presence of an inflamed or hemorrhagic Meckel diverticulum. In this situation, the Meckel diverticulum has a variable appearance and may simulate an inflamed duplication cyst, appendicitis, or sometimes a small intussusception. When one finds this somewhat atypical appearance on US, one should consider the diagnosis of a complicated Meckel diverticulum rather than the other pathologies it simulates ( Fig. 11.28 and Fig. 11.29 ; Video 11.28).


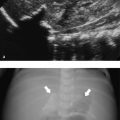
Necrotizing enterocolitis usually presents in infants in the neonatal intensive care unit, more commonly in premature neonates. The classic presentation includes abdominal distention and blood in the stool. The radiologist plays an important role at the time of the diagnosis of this condition, during follow-up, and in the detection of later complications such as strictures.
At the time of diagnosis, three abnormalities may be present on abdominal X-ray, which include bowel dilatation, intramural gas, and portal venous gas. Bowel dilatation is present in almost 100% of the patients with necrotizing enterocolitis, and the degree of bowel distention usually correlates well with the clinical severity. Follow-up abdominal X-ray may show asymmetric dilatation and fixed loops in those infants whose condition deteriorates. Intramural gas is not present in all patients, and the amount of intramural gas does not always correlate well with the clinical severity ( Fig. 11.30 ; Video 11.30). Portal venous gas usually is present in those with severe necrotizing enterocolitis ( Fig. 11.31 ). Disappearance of the intramural gas and portal venous gas does not always correlate with clinical improvement because the gas will eventually disappear even in those children who deteriorate clinically. Moreover, intramural gas is not pathognomonic for necrotizing enterocolitis because it can be seen in children with rotavirus or enterovirus enterocolitis. In fact, any severe colitis in combination with increased intraluminal pressure can produce pneumatosis and even portal gas ( Fig. 11.31 ; Video 11.31).


US is an extremely useful modality in the investigation of patients with necrotizing enterocolitis because it can provide information regarding bowel-wall thickness, bowel perfusion (color or power Doppler sonography), and the presence of intraperitoneal fluid. US is much more accurate than abdominal radiographs in documenting the presence of free and focal intraperitoneal fluid and can also define the character of such fluid. It is well-known that not all patients with necrotizing enterocolitis will show free air on abdominal radiographs following perforation and may present only with free fluid. Moreover, US in experienced hands can be extremely useful in detecting small amounts of free abdominal air, especially between the liver and anterior abdominal wall, in supine infants ( Fig. 11.32 ; Video 11.32).

In the early phases of necrotizing enterocolitis, the bowel wall will be quite thickened, but in those patients who are more severely affected, the mucosa and submucosa of the bowel slough into the lumen, leaving a markedly thinned bowel wall ( Fig. 11.33 ; also refer to Video 11.33). These patients are much more prone to perforation, and the thinning of the bowel wall can be documented with sonography. It is shown that in necrotizing enterocolitis the bowel (particularly thickened bowel) becomes markedly hyperemic, and this indicates the presence of viable bowel. However, the absence of perfusion in single or multiple loops of bowel (particularly when the bowel wall is thinned) indicates the presence of necrosis, and surgical intervention may be warranted even if there is no free air present on the plain radiograph.

US plays a major role in the follow-up of patients who are not responding to medical management and who deteriorate clinically. In these patients, US may provide information that is not depicted with abdominal X-ray.
Intestinal duplication cysts can occur anywhere along the alimentary tract, but the most common location is the ileum. Therefore, they are discussed in this section on the small bowel. Duplication cysts are spherical or tubular masses adherent to the gastrointestinal tract that sometimes communicate with it. They are lined with intestinal epithelium, and there is smooth muscle within the wall. The most common site is the ileum, followed by the stomach. Most patients present within the first year of life, with symptoms including gastrointestinal obstruction. Less common presentations include a palpable mass, intussusception, and abdominal distention.
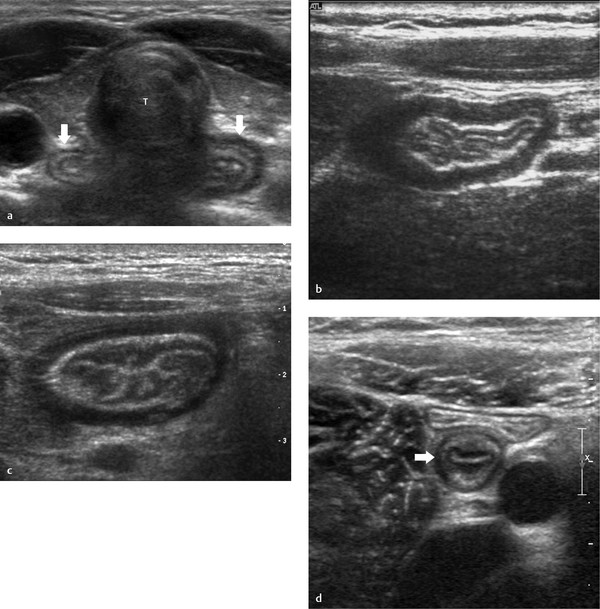
US easily appreciates the cystic nature of duplication cysts. The contents are often anechoic but may contain mucoid material or debris after hemorrhage. Rarely, a cyst appears completely hyperechoic after hemorrhage. Two signs are virtually diagnostic of duplication cysts: (1) a double-layered wall consisting of echogenic mucosa and hypoechoic muscularis propria ( Fig. 11.34 and Fig. 11.35 ) and (2) peristalsis in the cyst (Video 11.35). In 15 to 20% of cases, the cyst contains gastric mucosa, which causes the above-mentioned hemorrhages. Moreover, meticulous US examination may identify the primary bowel loop from which the cyst originates; the cyst shares the muscularis propria and serosa with the primary bowel loop ( Fig. 11.8 and Fig. 11.35 ). Ovarian cysts, urachal cysts, lymphatic malformations, and hemorrhage can all mimic duplication cysts ( Fig. 11.36 and Fig. 11.37 ).




Benign small-bowel intussusception is a recently described entity. It differs from classic symptomatic ileocolic intussusception in that benign small-bowel intussusception occurs predominantly in the right lower quadrant or periumbilical region, has a smaller diameter (mean diameter, 1.4 cm vs 2.5 cm) and a thinner outer rim, and does not contain mesenteric lymph nodes ( Fig. 11.38 ). Moreover, peristalsis in the intussuscepted loop persists, unlike in an ileocolic intussusception (Video 11.38). Often, benign small-bowel intussusception is an incidental finding, but it occurs with increased frequency and number in celiac disease ( Fig. 11.39 ). In general, benign small-bowel intussusceptions do not require immediate reduction because of their spontaneously resolving nature.


Children with ileocolic, ileo-ileocolic, or colocolic intussusceptions commonly do not present with the classic clinical triad of abdominal pain, red currant jelly stool, and a palpable abdominal mass. The presentation may therefore be nonspecific. For this reason, the clinician often has to rely on imaging procedures to diagnose or exclude intussusception promptly and accurately. The diagnosis can be made by ultrasonography, plain abdominal radiography, or contrast studies of the colon.
Ultrasonography has been shown in many series to be 100% accurate in depicting the presence or absence of the common types of ileocolic or ileo-ileocolic intussusception in children. These lesions have a characteristic sonographic appearance and are usually found just under the abdominal wall, most commonly on the right side of the abdomen ( Fig. 11.40 ). Because it is a noninvasive procedure and because of its accuracy, ultrasonography is the modality of choice for the evaluation of patients suspected of having an intussusception. An excellent review of the sonographic appearance of intussusception is provided in the article by delPozo et al.

Conventional abdominal radiography shows some characteristic signs of an intussusception, which include the meniscus sign, target sign, and less commonly a soft tissue mass. However, the sensitivity and specificity of conventional radiography are low. Only when there is a clinical consideration of peritonitis is the conventional radiograph essential to exclude perforation, which is the major contraindication to attempted enema reduction.
Pathologic lead points are found in about 5 to 7% of all intussusceptions. The most common are Meckel diverticulum, polyps, Henoch–Schönlein purpura, and cystic fibrosis. Less common causes are lymphoma, duplication cyst, and various inflammatory lesions of the bowel ( Fig. 11.41 ; Fig. 11.42 ; Fig. 11.43 ). Management of these patients remains a challenge.



Contrast or air enema techniques are not always diagnostic of a pathologic lead point. Sonography is extremely useful in this regard; it has been shown that it may depict two-thirds of pathologic lead points and may provide a specific diagnosis in one-third of these. However, it remains a diagnostic challenge as to how to search for a pathologic lead point when there is a high index of suspicion for such a lesion but ultrasonography is negative. In such cases, the choice of which other imaging modalities to use will depend on the individual patient’s clinical situation.
Many series in the recent literature have shown a reduction rate of intussusception varying from 80% to as high as 95%. These series have used either fluoroscopic or ultrasonographic guidance for reducing the intussusception and either hydrostatic (barium, water-soluble contrast, saline) or pneumatic reduction. The fact that different techniques have been used with similar success rates suggests that it is not important which technique is used. Nonoperative reduction of an intussusception should be attempted only after the surgical team has evaluated the patient and the patient is clinically stable and well hydrated, has no evidence of peritonitis, and has an intravenous line in place. The major contraindications to an enema are clinical findings of peritonitis or shock and signs of perforation on an abdominal radiograph. Absence of vascularization on color Doppler US and the presence of trapped fluid predict necrosis and/or unsuccessful hydrostatic reduction ( Fig. 11.44 ).

In order to improve one’s reduction rates, delayed or repeated attempts at reduction can be used as long as the intussusception does move during the initial attempted reduction and the child becomes asymptomatic and maintains stable vital signs. It has been shown that this approach is a safe and effective technique with a good success rate. Navarro et al used this approach in approximately 15% of patients with intussusception and achieved successful reduction in 50% of those intussusceptions not reduced on the first attempt. There does not appear to be a fixed optimal timing between attempts, and delayed second or third attempts can be made several hours after the first attempt.
Recently, US-guided external manual reduction was proposed as a safe and effective method to treat ileocolic intussusceptions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree