4 Spine
The use of ultrasound for imaging of the spinal canal in newborns and infants has been described in the literature since the beginning of 1980s. The spinal structures are superficially located in infants, and the dorsal structures of the vertebral column are mostly cartilaginous. This provides an acoustic window through which ultrasound can exquisitely demonstrate the spinal structures, especially with the use of a high-frequency (5- to 15-mHz) linear array probe. In children past the age of 6 months, progressive ossification of these dorsal structures obstructs the ultrasonic beam, and imaging becomes difficult or impossible. However, an impression of the position of the conus medullaris can be obtained in older children with a lower-frequency probe in the transverse plane.
Spinal dysraphism is defined as incomplete or absent fusion of midline neural, mesenchymal, and cutaneous structures. This leads to a wide spectrum of congenital spinal anomalies, ranging from a fatty-infiltrated filum terminale to a more extensive myelomeningocele. Some patients are completely asymptomatic, whereas others have bladder and bowel dysfunction, motor and sensory deficits, scoliosis, and secondary foot deformities. These deficits are often progressive.
Some authors believe that operative correction of a tethered cord can prevent the occurrence of neurologic deficit or halt further deterioration, whereas others are doubtful about the indication for untethering the conus medullaris in asymptomatic patients. Notwithstanding these differences of opinion, early detection of a tethered cord is worthwhile, and knowledge of its presence can help to plan future management. The main role of ultrasound is as a screening examination for occult spinal dysraphism. If ultrasound shows a normal position of the conus medullaris and an absence of lower intraspinal anomalies, then magnetic resonance (MR) imaging is not indicated. If ultrasound does show anomalies, an MR imaging examination will generally be done to confirm the diagnosis, and also to obtain baseline images for future comparison once ultrasound is no longer possible because of progressive ossification of the vertebrae. MR imaging is also indicated when a discrepancy exists between the clinical signs and symptoms and the results of ultrasound examination.
The main indications for spinal ultrasound can be divided in two categories:
Screening the vertebral column for anomalies in infants with other congenital anomalies that are associated with occult spinal dysraphism, like anorectal and cloacal malformations;
Examining the spine of infants with a back mass or cutaneous markers, the latter of which include skin hemangiomas, sinuses, dimples, and abnormal hairy patches.
Perhaps ultrasound of the vertebral column is most frequently requested for the infant with a sacrococcygeal dimple. If the dimple is over the distal coccyx, then intraspinal pathology is extremely rare. If the dimple is higher up, and especially if it is combined with skin anomalies or an abnormal gluteal cleft, intraspinal anomalies are much more likely to be present.
Spinal ultrasound is also described for other indications, including ultrasound-guided lumbar puncture, intraoperative ultrasound, and ultrasound in diagnosing traumatic lesions of the spinal cord.
The anomalies of the vertebral column can be divided into three categories:
Non skin-covered (open) back masses (e.g., myelocele and myelomeningocele);
Skin-covered (closed) back masses (e.g., meningocele, lipomyelocele, lipomyelomeningocele, and myelocystocele);
Occult spinal dysraphism (e.g., intradural lipoma, dorsal dermal sinus, syringohydromyelia, diastematomyelia, caudal regression syndrome, anterior sacral meningocele, and tight filum terminale).
In non skin-covered back masses, ultrasound plays no clinical role. The diagnosis is obvious, and an ultrasound examination could lead to infection of the exposed neural tissue.
In skin-covered back masses, ultrasound can be useful as a quick orientation, but MR imaging will still need to be performed. The ultrasound will help the clinician decide if there is involvement of neural tissue, as in a myelocystocele or lipomyelomeningocele, as opposed to a simple meningocele. MR imaging and ultrasound provide summative information in this setting.
It is in the third category, suspected occult spinal dysraphism, that ultrasound can be most useful. Ultrasound can often make a proper diagnosis and help the clinician to decide on the timing of an additional MR imaging examination, if required.
Motion-mode (M-mode) ultrasound can help add further information. It can quantify the anteroposterior oscillation of the spinal cord and cauda equina with the cardiac cycle.
4.1 Embryology
In this section, the embryologic development of the spinal canal is briefly described.
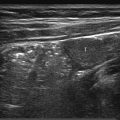
The notochord forms between the 18th and 20th day of embryologic development. During primary neurulation, the notochord induces the formation of a neural plate from the dorsal ectoderm, which then forms the neural groove ( Fig. 4.1a). This groove deepens, folds in, and forms the neural tube (which is an ectodermal structure; Fig. 4.1 b). Subsequently during disjunction, the neural tube separates from the cutaneous ectoderm when a layer of mesenchymal cells is interposed between the neural tube and the cutaneous ectoderm. Closure of the neural tube starts at the 22nd day at the level of the future junction of the brain and spinal cord. From here, the tube proceeds in closing in both rostral and caudal directions.

Secondary neurulation is a process that starts at the caudal end in the 22-day embryo. A mass of mesodermal cells, the caudal cell mass, is formed. This then expands to form the distal part of the notochord and the spinal cord, forming the neural cord. The lumen of the primary neural tube invaginates into this neural cord, forming the primitive spinal cord. At the terminal end of the spinal cord, the central canal subsequently widens to form the terminal ventricle. Around the 50th day of embryologic development, through a process called retrogressive differentiation, the caudal cell mass diminishes in size and the distal spinal cord involutes. Secondary neurulation leads to the formation of the conus medullaris and the more caudal spinal canal, including the filum terminale.
A deficient primary neurulation with nonclosure or reopening of the distal neural tube will cause a myelocele or myelomeningocele. Premature disjunction of the cutaneous and neural ectoderm will cause mesenchymal cells to come into contact with the nonneurulated neural plate. The mesenchymal cells differentiate into fat cells, which adhere to the neural plate, forming a lipomyelomeningocele or an intradural lipoma.
Disorders of secondary neurulation lead to a variety of congenital malformations. Lipomas of the filum terminale and a tight filum terminale are caused by disorders of retrogressive differentiation. Malformation of the terminal ventricle can produce a cystic dilatation of the distal cord or a distal myelocystocele. Malformation of the sacrum can lead to an anterior sacral meningocele. Excessive retrogressive differentiation causes caudal regression syndrome with underdevelopment of the distal spinal column, malformation of the distal conus medullaris, and associated abnormalities of the distal colon and urogenital tract.
4.1.1 Ascensus Medullaris
During embryologic development, the conus medullaris ascends from the distal vertebral column to its normal position at the level of the first to second lumbar vertebrae. This is probably caused by a disproportionate growth between the vertebral column and the neural tube. The nerve roots of the lower spinal cord segments emerge from the spinal canal at the foramina of the corresponding vertebrae. This results in the formation of the cauda equine, which is a bundle of nerve roots distal to the conus medullaris in the subarachnoid space.
Between 13 and 18 weeks of gestation, the conus medullaris is situated at the level of the L4 vertebra, or more caudally, in 100% of fetuses. At 27 weeks, the conus medullaris is on average at the L2–L3 level. At term, the conus medullaris is often at its “adult” position at the L1–L2 level, but the position is considered normal up to the L2–3 disk level.
4.2 Technique of Spinal Ultrasound
Spinal ultrasound is easy and quick to perform. Unrest and lack of cooperation on the part of the patient, although increasing the challenge, will hardly ever cause an unsuccessful examination.
The infant can be positioned in the prone or in the lateral decubitus position. The latter is favored because it is easier to immobilize the infant. In a prone position, older children tend to arch the back, hindering good contact between the probe and the skin. If a small posterior meningocele is suspected, the infant can be examined in an upright position, which can distend the meningocele with fluid.
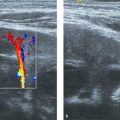
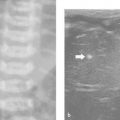
Sagittal images provide a nice view of the spinal cord and the distal nerve roots. A panoramic view (when this option is available) will provide an extended overview and facilitate the determination of the position of the conus medullaris ( Fig. 4.2 ). Transverse images are favored to show the nerve roots and assess the filum terminale for thickening. Most other anomalies are also easier to diagnose on transverse images than on sagittal images.

The first cardinal question to answer when scanning is to identify with certainty the location of the conus medullaris, the lower, slightly bulbous end of the spinal cord. There are several methods to assess this position.
With some practice, the lumbosacral junction (the transition from the lumbar vertebrae to the sacrum) can be reliably located by identifying the change in angulation of the spine at this level. Counting the vertebral bodies upward from the lumbosacral junction will then allow correct localization of the level of the conus medullaris (see Fig. 4.2 ).
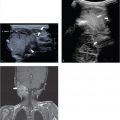
A complementary method is to locate the last rib (presumed 12th rib) and through this the T12 vertebral body. This is done by positioning the probe in a parasagittal plane to identify the echogenic short last rib superficial to the kidney. The rib is then followed medially to the T12 vertebral body ( Fig. 4.3 ).

As a method of last resort, one can sonographically locate the iliac crest, which should be anatomically located at the level of the fifth lumbar vertebra.
All the above techniques should come to the same conclusion and position the conus medullaris at the same vertebral level. Anatomical variants and anatomical anomalies (e.g., 11 or 13 ribs, increased/decreased numbers of lumbar vertebrae, and segmentation anomalies in the lower spine) can complicate the above assessments, resulting in conflicting results.
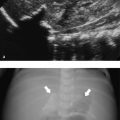
If the above methods are unsuccessful or give indeterminate/conflicting results, the sonographer can count upward from the lowest ossified sacral or coccygeal vertebral body, place a radiopaque marker at the level of the conus medullaris, and obtain a lateral plain film of the vertebral column. In this way, the position of the conus medullaris can be determined. It is important to appreciate that ultrasound is very sensitive for bony structures and can detect tiny ossification centers that are not well appreciated on plain films. The sizes of the ossification centers seen on plain film and ultrasound should, however, more or less correspond.
After the location of the conus has been assessed, attention should shift to the morphology and integrity of the cord. When the scan is being performed for a sacral dimple, only the lumbar cord needs assessment. If a more complex abnormality is being assessed, then it would be best examine the entire cord up the cervical region.
Following this, the subarachnoid space and the distal nerve roots should be assessed. One should confirm the presence of normal pulsatile movements in the distal nerve roots and cauda equina. If the child is in a decubitus position, the nerve roots can gravitate to the dependent side ( Fig. 4.4 ).

The filum terminale needs to be assessed separately in transverse and sagittal planes. The lower vertebral column and the posterior elements should be carefully reviewed, with special attention paid to the presence and anatomy of the sacrum and the coccyx.
M-mode sonography is described in the literature as a useful tool to assess the free pulsatile movement of the cord and nerve roots. However, in many normal infants, M-mode may show no movement at all. Additional information that can be provided by M-mode sonography includes quantification of the anteroposterior oscillation of the spinal cord and cauda equina. An oscillation amplitude of less than 0.3 mm in a symptomatic patient should alert the clinician to cord abnormalities and/or tethering.
With color Doppler, the venous epidural plexus can be seen, and sometimes the spinal arteries are also visualized.
Tips from the Pro
Always use a linear probe with the highest frequency available. If you detect any abnormality, extend the examination along all the visible spinal cord, and also review the kidneys in the same study.
4.3 Normal Sonographic Anatomy
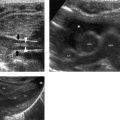
When examined in the sagittal plane, the spinal cord is depicted as a hypoechoic to anechoic tapering structure with a central echo complex. This linear echogenic appearance is probably secondary to reflection from the central canal. Another possible explanation is that this increased central echogenicity is caused by the central end of the fissura mediana anterior. With current techniques, differentiation between gray and white matter is not yet possible. The margins of the spinal cord appear echogenic, and the cord is surrounded by echogenic spinal nerve roots. The distal conus medullaris is thicker than the more proximal thoracic cord ( Fig. 4.5 ).

The tip of the conus medullaris is usually located at the L1–L2 level, but a position down to the L2–3 disk space is considered normal.
On transverse images, the conus medullaris is oval, with its transverse diameter larger than its anteroposterior depth ( Fig. 4.6 ). Thin, echogenic strands of the arachnoid can be seen, and larger echogenic dots representing the nerve roots are well identified laterally at this level. At the more proximal thoracic level, the dentate ligaments can also be visualized as echogenic lines at the lateral margins of the cord. These ligaments are not as well identified at the level of the conus medullaris.

Below the conus medullaris, the grouped nerve roots constitute the cauda equina ( Fig. 4.7 ). The number of nerve roots diminishes caudally as two ventral and two dorsal nerve roots exit at every vertebral level. The dural sac ends at the level of the second sacral vertebra.

The filum terminale is in continuity with the tip of the conus medullaris and is depicted as a centrally located, slightly echogenic structure ( Fig. 4.8 ). It can be followed to the caudal end of the dural sac. It attaches to the coccyx, but below the dural sac the filum terminale is not visible as a separate structure. This structure should be less than 2 mm in axial diameter. Its echogenicity needs to be carefully assessed to exclude fatty contents within it.

The bony structures and vertebral bodies are visualized as echogenic structures. The intervertebral disks appear hypoechoic. The cartilaginous, unossified parts of the vertebra, especially the spinous processes, are hypoechoic or anechoic, increasing in echogenicity with age.
The first coccygeal vertebra can be ossified in the young infant. The distal coccyx is composed of anechoic to hypoechoic cartilage. The coccyx generally has the same continuous sacral kyphotic curve as in adults, although it is occasionally straight. The tip of the coccyx can even have a lordotic curvature (upturned toward the skin/probe) and present as a small, palpable “distal mass” ( Fig. 4.9 ).

4.3.1 Normal Variants
A slight widening of the central echo complex can be seen in normal infants. No upper limits of normal are available ( Fig. 4.10 ).

Sometimes, a more local dilatation of the distal central canal in the conus medullaris is seen. This is called the ventriculus terminalis. It is considered a normal variant if it is anechoic, not septate, and not associated with any other findings. It should measure less than 4 mm in diameter ( Fig. 4.11 ).

A filar cyst is a small, cystic, midline anechoic structure that can be seen in the proximal filum terminale. This has no clinical significance ( Fig. 4.12 ).

A pseudosinus tract is a residual cordlike structure occasionally seen extending from the tip of the coccyx to an adjacent deep end of a sacral dimple ( Fig. 4.13 ). A dermal sinus is located more proximally.

Acceptable variety in the contour of the coccyx has been described in the preceding section.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree