17 Musculoskeletal Ultrasound
17.1 Pediatric Hip
Ultrasound (US) has added substantially to the management of hip disorders in newborns and infants, particularly with respect to developmental dysplasia of the hip, and also to the different forms of arthritis.
17.1.1 Normal Development of the Hip
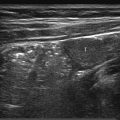
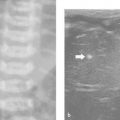
The hip joint forms by cleavage after 8 weeks’ gestational age. The fetal period is characterized by further growth and early endochondral ossification of the iliac, ischial, and pubic nuclei. These nuclei subsequently fuse, although separated by a growth zone (the Y-cartilage), to form the bony part of the hip socket. At birth, the shape of the bony socket varies along a continuum, from a smooth, round acetabulum with a well defined lateral edge (85% of all newborns) to a shallow, dysplastic acetabulum (3 to 4% of all newborns). This is best demonstrated on a coronal ultrasound view through the mid acetabulum (Graf standard view; Fig. 17.1 ). During the next few months, as the hyaline cartilage ossifies, the socket takes on a more mature shape, provided that the femoral head is stable and well centered. Around 1 to 2% of all newborns require abduction treatment to facilitate the maturation process. This figure rises to 6 to 7% according to the definition used for pathology.

The normal femoral head is not strictly spherical, but slightly ovoid. The ossification center is visible sonographically in 3% of all newborns and normally appears radiologically at 3 to 4 months of age, somewhat earlier in girls than in boys. By 8 months of age in girls and 10 months in boys, the ossification center is visible in nearly 100% of normal hips. Appearance after the age of 10 months is considered pathologic, and conditions such as Meyer dysplasia and multiple epiphyseal dysplasia should be considered in the differential diagnosis. During the first year of life, the sizes of the ossification centers may differ slightly between the two sides.
At about 8 to 9 years of age, secondary ossification centers appear at the acetabular margin. Presumably, these centers are of importance for further growth of the acetabulum, and they subsequently fuse with the pelvic bone at about 18 years of age. In adults, the acetabulum covers slightly more than 50% of the femoral head.
17.1.2 Ultrasound Examination for Developmental Dysplasia of the Hip
Developmental dysplasia of the hip (DDH) is the most common musculoskeletal disorder in infancy, with a reported prevalence from 0.5 to 4% according to the case definition used, age, ethnicity, and method of ascertainment. The term developmental dysplasia of the hip refers to a spectrum of pathology, ranging from the normal to severely dysplastic hip, with or without coexisting instability, in the newborn to the dysplastic and dislocated hip presenting in later infancy or early childhood. DDH affects both hips in approximately 30 to 40% of the cases; in unilateral disease, the left hip is more often affected than the right. More girls are affected than boys; for DDH presenting in later childhood, the ratio of girls to boys is approximately 5:1. Similarly, DDH detected on ultrasound, based on a modified Graf technique (Rosendahl), is also more common in newborn girls, affecting 5.7% of all girls compared with 1.2% of boys. At skeletal maturity, the prevalence of hip dysplasia is reported at 3 to 19%, again according to the case definition used, age, ethnicity, and method of ascertainment. DDH is the underlying mechanism contributing to nearly one-fourth of hip-joint replacements in adults ages 40 years and younger.
Ultrasound Technique
A combined technique for assessing both acetabular morphology and hip stability is advised. Although there is a strong association between hip morphology and stability, morphologically normal hips may be unstable, and vice versa. Hip morphology should be assessed with a Graf coronal (standard) section through the mid acetabulum, whereas hip stability may be assessed with different views (coronal, axial).
We examine the child in the lateral position, placed in a special, self-made hip cradle for stabilization ( Fig. 17.2 ). Hip morphology is assessed with the baby’s hip slightly flexed and in a neutral abduction–adduction position to avoid lateralization in cases of instability. The baby’s femur is held with the examiner’s right hand, while the left hand holds the transducer, with the fourth and fifth fingers sliding behind the sacrum to stabilize the pelvis ( Fig. 17.2 ). The transducer, preferably a high-frequency (5–17 MHz as appropriate for the baby’s size) linear probe, is placed along the hip joint, perpendicular to the skin. After the caudal portion of the iliac bone has been identified abutting the Y-cartilage, the transducer is gently rotated until the distal part of the iliac bone parallels the contour of the image ( Fig. 17.3 a). The resultant image represents the coronal (standard) view ( Fig. 17.1 ). After having secured the standard view with the femoral head centered in the acetabulum, we perform a Barlow-like provocation test with the baby and the examiner’s hands in the same starting position. The few hips that are irreducible are assessed with a dislocated femoral head.


The most accurate approach for defining and grouping morphology is to measure the acetabular α-angle, or bony inclination, on a coronal midacetabular view ( Fig. 17.3 a). The α-angle is the equivalent of the acetabular index on a pelvic radiograph and is based on lines drawn between fixed reference points for increased accuracy. In contrast, the assessment of femoral head coverage is based on at least one movable reference point, such as the capsule ( Fig. 17.3 b). In cases of hip instability, which is seen in up to 2% of all newborns, this measurement technique may bias the result significantly. Most hips stabilize during the first couple of weeks; however, instability, although subtle, can be seen up to the age of 3 to 4 months, or even longer.
Hip stability is best assessed sonographically because the clinical Barlow–Ortolani tests are relatively inaccurate. Different US techniques have been used, including the Harcke dynamic, multiplanar approach. We prefer to perform the provocation test after the assessment of hip morphology, with the baby in the same lateral position ( Fig. 17.2 ). With the right hand, the baby’s hip is slightly rotated inward while mild pressure is applied along the axis of the femur (i.e., a telescopic movement). If no instability can be elicited, the same maneuver can be repeated three to four times with slightly different degrees of inward rotation. Because a screaming, distressed baby can hinder the detection of an unstable hip, we opt for a baby-friendly environment, including warm US gel and warm hands. In special cases, we even use a pacifier dipped in 50% sucrose or give 1 to 2 mL of 5% glucose per orally, in accordance with routines commonly used for premature babies.
Hip Classification: Morphology and Stability
Based on hip morphology and the α-angle, hips are classified as normal (α-angle ≥ 60 degrees), immature (50 degrees ≥ α-angle < 60 degrees), or dysplastic (mild, 43 degrees ≥ α-angle < 50 degrees; significant, α-angle < 43 degrees), with or without instability ( Fig. 17.4 ). In regard to stability, hips are classified as stable, unstable (but not dislocatable), dislocatable (Barlow+), or dislocated (Ortolani+; Fig. 17.5 ). When a hip is dislocated, traction and abduction are performed to test whether or not the femoral head is reducible ( Fig. 17.6 ). A partly reducible hip with a bright acetabular cartilage often is associated with delayed ossification or a dysplastic acetabulum ( Fig. 17.7 ), although the hyperechogenicity can disappear after reduction of the femoral head ( Fig. 17.8 ). Early surgical intervention to restore containment should be considered in these cases.





The results should be reported in a standardized way, including the indications for the examination, the clinical findings (if possible), the US findings for each of the hips separately, and recommendations for further management.
Clinical Validity of the Findings
During the last 25 years, the significance of acetabular dysplasia as described by Graf has been examined in a substantial number of studies. At birth, the mean acetabular inclination angle (α-angle) is 62.5 degrees (standard deviation [SD], 5.9) for girls and 65.4 degrees (SD, 5.1) for boys ( Fig. 17.9 ). From population-based studies, we have learned that around 75 to 85% of newborns have morphologically normal hips, 13 to 25% have immature hips, and 2 to 4% have dysplastic hips. One study reports a strong association between hip morphology and stability; only 0.1% of morphologically normal hips are dislocatable, versus 0.6% of immature hips, 62% of slightly dysplastic hips, and almost 100% of severely dysplastic hips. Several studies show that morphologically normal hips tend to remain normal with or without coexisting instability and that 97% of sonographically immature hips tend to normalize spontaneously within 3 months; a similar pattern has been observed for mildly dysplastic but stable hips. A recent 6-year follow-up of infants with mildly dysplastic and potentially unstable hips showed no difference in radiographic outcome at 6 years of age between those allocated to initial splintage for 6 weeks and those offered active sonographic surveillance. The delayed acetabular ossification or persistent dysplasia seen in a third of infants from both groups at 1 year of age had completely resolved in all but one of the girls in the treatment group.

Tips from the Pro
A calm baby is crucial for successful ultrasound of the hip. Use sugar if necessary!
A correct standard section is key to a correct diagnosis.
In newborns, a dislocated hip that is not entirely reducible requires early treatment to reduce or prevent damage to the acetabular cartilage.
17.2 Ultrasound of the Musculoskeletal System in the Older Child
17.2.1 Arthritis
Arthritis may result from or be associated with a number of conditions, including connective tissue diseases, trauma, congenital disorders, inflammatory diseases, and hematologic and neoplastic diseases. Here, we will focus on inflammatory disease and juvenile idiopathic arthritis but also comment on some of the other diseases that can be associated with joint symptoms in children.
Transient Synovitis of the Hip
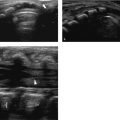
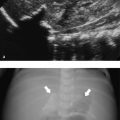
Transient synovitis of the hip is an inflammatory condition of unknown origin that affects children between 2 and 10 years of age. Children typically present with an acute onset of local pain and a limp. The condition is self-limiting, and most patients recover after 1 to 3 weeks without any sequelae. A complicating Perthes disease has been reported in 1 to 2% of cases. Boys are more frequently affected than girls. The history and clinical and laboratory findings may help to rule out the differentials, in particular septic arthritis and trauma. US is the preferred imaging method, showing an anechoic effusion without any significant synovial thickening ( Fig. 17.10 and Fig. 17.11 ). If the findings are equivocal, US-guided aspiration should be considered. If there is no improvement within 3 weeks, additional imaging is indicated. Radiography can be used to rule out Perthes disease; if the results are normal, magnetic resonance (MR) imaging can be helpful.


Septic Arthritis
Septic arthritis is seen more frequently in the pediatric population than in adults. In neonates, extension of a metaphyseal focus of osteomyelitis is a common route of infection, whereas hematogenous spread is more likely in older children. At all ages, there is a great diversity of infecting bacteria, but Staphylococcus and Haemophilus influenzae predominate in infants younger than 2 years of age. The hip is most frequently affected, especially in neonates. The diagnosis is based on joint aspiration, US-guided if possible. On US there is an effusion that often has echogenic material within ( Fig. 17.12 ). The capsule and surrounding tissues can be thickened. If a complicating abscess is suspected, MR imaging should be considered to guide therapy.

Juvenile Idiopathic Arthritis
Juvenile idiopathic arthritis (JIA) is a heterogeneous condition encompassing all forms of chronic arthritis of unknown origin that has an onset before 16 years of age. It is characterized by chronic synovial inflammation, with a risk for the development of progressive joint destruction and serious functional disability. The reported incidence varies from 0.6 to 1 in 1,000 children, so JIA represents an important cause of acquired disability in children. During the last decade, new and potent therapeutic agents have become available to children with JIA, underscoring the need for accurate monitoring of the therapeutic response in regard to both disease activity and structural damage to the joint, prevention of the latter being considered the gold standard in studies of treatment efficacy. Current classifications, based on clinical criteria, are unsatisfactory because clinical parameters are poor markers of disease activity/progress and joint destruction.
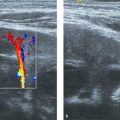
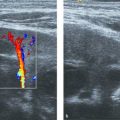
The role of imaging in JIA is first to diagnose the disease, second to evaluate disease activity, and third to monitor the effect of therapy. There has been a shift from traditional radiography toward newer techniques such as US and MR imaging, without proper evaluation of their accuracy and validity. Joint damage in JIA is traditionally evaluated with radiographic methods of scoring joint space narrowing and erosions; however, these are quite insensitive, in part because of the growing skeleton. Wrist disease has been associated with a more severe course of arthritis and a poorer functional outcome, and the wrist is the only joint for which suitable radiographic measures of disease progression have been reported. Much effort has been expended recently on validating existing scoring systems and devising new ones, of which the adapted versions of the Sharp/van der Heijde score have gained the most widespread acceptance.
US is superior to clinical examination in the diagnosis and localization of joint effusion, bursal fluid collection, and synovitis ( Fig. 17.13 ). MR imaging is superior to other modalities in showing subtle cartilage and soft-tissue changes in the early stages of the disease, when radiographs are still normal. Computed tomography (CT) may be of value in visualizing the axial skeleton and sacroiliac joints. Bone scintigraphy may be useful in evaluating disease activity; however, the method is flawed, yielding both false-positive and false-negative results.

Ultrasound Techniques for the Assessment of Joints
US is performed with a high-frequency linear transducer (12–17 MHz), and the child is positioned according to which joint is being examined. The procedure must be tailored to the patient’s complaints and the location of pathology, and it should include a dynamic assessment and Doppler imaging when necessary. Below, we present the commonly used approaches.
Hip
With the child supine, hips extended, and in a neutral rotational position, the transducer is placed along the femoral neck to produce the standard view ( Fig. 17.14 ). This includes the femoral head and neck, the capsule with its insertion, and the iliopsoas muscle. The capsule normally parallels the femoral neck from its origin at the outer surface of the labrum to its insertion at the intertrochanteric line. Here, it merges with the periosteum, although some fibers turn back to form an inner layer, inserting at the junction between the articular cartilage and bone. Both the outer and the inner capsular layers are lined with a thin synovial membrane comprising only one to three cell layers. On US, a thin, bright line representing the interface between the two layers can be seen in about 70% of normal hips ( Fig. 17.14 ). Moreover, in normal hips, differentiation between the synovial membrane and the fibrous capsule is impossible. Most often, both capsular layers have similar echogenicity and are isoechoic to the psoas muscle in about half of children and hyperechoic in one-third. In one-fifth of hips, the outer capsular layer is brighter than the inner ( Fig. 17.14 ). The thickness of the joint capsule as measured from its outer contour to the femoral neck is usually 4 to 5 mm, with no significant differences between left and right. A tiny sliver of joint fluid can be seen in approximately 6% of healthy children. If no fluid can be seen with the hip in the neutral position, additional images with the hip in internal and external rotation should be obtained. Small effusions will then become visible. Note that on internal rotation, the capsule normally bulges slightly. A second view, including the femoral head and the labrum, should be obtained to evaluate the sphericity of the femoral head and the labrum ( Fig. 17.15 ). (Perthes – slipped capital femoral epiphysis – labral pathology?).


Knee
The child is supine, with a slightly flexed knee joint to avoid biasing (e.g., anisotropy) of the quadriceps and patellar tendons. The standard sagittal view includes the patella inferiorly, the quadriceps tendon, and the suprapatellar recess ( Fig. 17.16 ). The recess is an acoustic window into the knee joint. If no fluid is seen on the initial scan, additional views obtained under manual compression (i.e., with a maneuver similar to that used for the “patellar dip” test) will lead a small effusion into the recess. On the other hand, if the recess is distended, graded compression with the transducer will help distinguish an effusion from synovial hypertrophy ( Fig. 17.17 ). Normally, a sliver of anechoic fluid can be found at all ages. An axial view in the popliteal fossa is helpful to exclude a Baker cyst.


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree