14 Adrenal Glands
Ultrasound (US) is the primary imaging modality employed to evaluate the abdomen in infants and children. With respect to adrenal pathology, a palpable mass may be confirmed as having an adrenal origin, or an adrenal lesion may be actively searched for in a child who presents with a hypertensive or metabolic crisis, an endocrinopathy, or neurologic symptoms and signs. Asymptomatic adrenal masses may be detected when the abdomen is being examined for some other reason.
The radiologist must describe any mass lesion identified, determine whether an abnormality is unilateral or bilateral, and document any further intra-abdominal foci of disease. Many acquired forms of adrenal pathology appear similar on US, and more detailed anatomical information and tissue characterization with computed tomography (CT) or magnetic resonance (MR) imaging is often required. Radioactive isotope examinations with iodine I 123 metaiodobenzylguanidine (123I-MIBG) and/or technetium Tc 99m methylene diphosphonate (99mTc-MDP), and functional studies with fluorodeoxyglucose F 18 positron emission tomography (18F-FDG-PET), are employed in those children with known or suspected adrenal malignant disease. Correlating the imaging findings with the available clinical and biochemical information is crucial in the context of adrenal disease.
Tips from the Pro
The majority of incidentally detected adrenal masses in children is malignant, and with the exception of small lesions detected in infants younger than 3 months of age, in whom judicious observation may be employed, all should be resected.
14.1 Embryology of the Adrenal Glands
The adrenal glands are composed of a separate outer cortex and an inner medulla, which have different embryologic origins and postnatal functions.
The fetal (primitive) cortex develops from the coelomic mesoderm of the urogenital ridge on the posterior abdominal wall during the fifth gestational week. In the seventh week, these cells are surrounded by additional mesothelial tissue as the adult cortex is formed. This mass of cortical cells separates from the adjacent mesothelial tissue in the eighth week and becomes enveloped in connective tissue. The adrenal medulla is formed later, when chromaffin cells derived from the sympathetic ganglia of the neural crest invade the medial aspect of the cortex. The central location of the medullary cells is achieved by the 18th week. At birth, the adrenal glands are large relative to the kidneys, but there is rapid postnatal involution of the fetal cortex, so that by 2 weeks of age, the adrenals have lost more than 30% of their birth weight.
The histologic and functional development of the adrenal glands continues throughout infancy, with the cortical zona glomerulosa, zona fasciculata, and zona reticularis fully differentiated by 3 years of age. Aldosterone, produced in the outermost zona glomerulosa layer of the cortex, plays an important role in salt–water balance as part of the renin–angiotensin–aldosterone system. A complex negative feedback mechanism of the hypothalamic–pituitary–adrenal axis regulates adrenal cortical endocrine function postnatally. Cortisol production predominates in the zona fasciculata (middle layer), with adrenal androgens arising in the inner zona reticularis.
14.2 Normal Anatomy
The adrenal glands are paired retroperitoneal organs that lie within the Gerota fascia in close relation to the kidneys. On the right side, the adrenal lies posterior to the inferior vena cava (IVC) and superomedial to the upper pole of the right kidney. The left adrenal lies more anteriorly and inferiorly to the kidney than does the right adrenal. Immediately adjacent to the left adrenal are the aorta medially and the pancreas and splenic vein anteriorly. The right adrenal has a pyramidal shape; the contour of the left-sided gland is slightly flatter and is described as semilunar in shape.
The glands have a rich arterial supply from the superior, middle, and inferior adrenal arteries. Respectively, these paired vessels arise from the inferior phrenic artery (a branch of the descending aorta), the abdominal aorta, and the ipsilateral main or upper pole renal artery. Usually, each gland is drained by a single vein that emerges from the hilum. On the right side, the vein communicates with the posterior aspect of the IVC, whereas the left-sided vein unites with the left renal vein.
14.3 Normal Sonographic Appearance
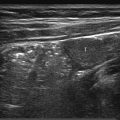
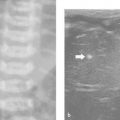
The normal neonatal adrenal is clearly identified with US. The histologically distinct cortex is viewed as a thick, echo-poor outer layer that surrounds a thinner echo-bright vascular core of medullary tissue ( Fig. 14.1 ). The combination of a relatively large size (the neonatal adrenal is about one-third as long as the adjacent kidney) and a lack of retroperitoneal fat aids visualization. With age, the layered appearance of the gland is lost, and a normal adrenal in an older child and adult is seen merely as an echogenic “triangle” atop the kidney below ( Fig. 14.2 and Fig. 14.3 ).



Tips from the Pro
A high-frequency, 6.0- to 8.0-MHz probe is used to evaluate the neonatal adrenal gland. The use of a linear transducer can further enhance the images.
14.4 Normal Variants
Congenital anomalies of the adrenal glands are uncommon and are usually diagnosed incidentally or during renal sonography in infants with other medical or surgical problems. The variant most frequently visualized by a pediatric sonographer is the “lying down” adrenal, encountered when the adjacent kidney is ptotic or absent ( Fig. 14.4 ).

Midline fusion of the adrenal glands (“horseshoe” adrenal) is well documented, but rare ( Fig. 14.5 ). The sonographic architecture is maintained, along with gland function. The majority of cases is seen in infants with asplenia (Ivemark syndrome).

If fragments of adrenal tissue break off during development, then cortical rests or true accessory adrenal glands (cortical and medullary components) develop, depending upon the timing of the event during gestation. Accessory adrenal tissue may be found around the celiac axis, close to the normal location of the gland. Descent of cortical tissue into the lower abdomen and pelvis at the time of gonadal migration explains descriptions of adrenal tissue within the uterus, broad ligament, ovary, inguinal canal, and scrotum. Accessory adrenal tissue may be hormonally active and become hypertrophic under the influence of adrenocorticotropic hormone (ACTH), most notably in patients with undertreated congenital adrenal hyperplasia. Testicular adrenal rests are seen in the majority of male patients with this condition and are reported as well defined, hypoechoic, intratesticular lesions. They may be bilateral and are often impalpable.
Tips from the Pro
Do not confuse a normal neonatal adrenal gland for a dysplastic kidney in cases of renal agenesis or ptosis.
14.5 Pathology
14.5.1 Neonatal Adrenal Hemorrhage
Neonatal adrenal hemorrhage is seen in association with birth trauma, perinatal asphyxia, and septicemia. Less commonly, hemorrhage may be found in babies with bleeding diatheses. The cause is not known, but increased adrenal arterial flow or elevated venous pressures likely contribute. Unilateral hemorrhage contained within the gland is generally asymptomatic, but extracapsular extension can progress to severe hypovolemia and even death. Anemia, jaundice, and abdominal and scrotal masses are all reported. Adrenal insufficiency is a theoretical possibility when there is bilateral extensive hemorrhage.
The sonographic appearance depends upon the age and size of the hematoma. Early on, the mass may be iso- or hyperechoic in comparison with the surrounding tissues ( Fig. 14.6 a). Gradual liquefaction and shrinkage occur over the following weeks ( Fig. 14.6 b–d). By 3 months of age, most adrenal hemorrhages have resorbed completely or have calcified. In the latter case, the hemorrhage is echo-bright and may have posterior acoustic shadowing.

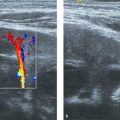
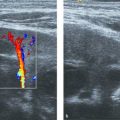
The differential diagnosis for a neonatal adrenal mass includes congenital neuroblastoma ( Fig. 14.7 a), simple adrenal cyst ( Fig. 14.7 b), obstructed upper moiety of a duplex kidney ( Fig. 14.7 c), and enteric duplication cyst ( Fig. 14.7 d).

Tips from the Pro
Differentiating an adrenal hemorrhage from a congenital neuroblastoma is difficult. Nevertheless, the following features suggest malignancy rather than hemorrhage: antenatal detection of the mass, foci of calcification seen on initial assessment, internal vascularity by color Doppler, and gradual evolution to a more complex-appearing mass.
14.5.2 Adrenal Hemorrhage in the Older Child
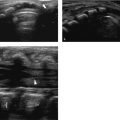
Adrenal hemorrhage is well recognized in children following blunt abdominal trauma. It is more commonly identified on the right side and is observed in conjunction with injuries to ipsilateral solid organs and the lower chest. Spinal trauma and diaphragmatic rupture are also documented with adrenal hemorrhage. The diagnosis is usually incidentally noted during CT examination of a severely injured child.
Hemorrhage into a preexisting tumor is known to occur, particularly with hypervascular lesions such as pheochromocytoma, angiomyolipoma, myelolipoma, and hemangioma. Adrenal hemorrhage in children receiving extracorporeal membrane oxygenation (ECMO) therapy and secondary to renal vein thrombosis is also recognized.
Tips from the Pro
When an adrenal hematoma is identified in an older child with no documented history of trauma, nonaccidental injury should be considered in the differential diagnosis ( Fig. 14.8 ).

14.5.3 Adrenal Cysts
Adrenal cysts are often found incidentally when the abdomen is imaged for some other reason. They are subclassified into endothelial, epithelial, and pseudocyst types; the latter are more common and are due to hemorrhage, infarction, tumor, or dysplasia. Multiple microcysts or macrocysts can be found in some patients with Beckwith–Wiedemann syndrome.
Endothelial cysts include lymphangioma and hemangioma. Epithelial cysts include congenital cysts and those that arise secondary to infection with parasites such as Echinococcus (hydatid disease). Hemorrhage and infection may complicate an adrenal cyst.
At sonography, cysts may be unilateral or bilateral, single or multiple, simple or complex ( Fig. 14.9 ). Occasionally, their walls may show thin rims of calcification.

The clinical history is very important in distinguishing between the subtypes.
14.5.4 Adrenal Abscesses
Adrenal abscesses are rare. An abscess may develop as a primary lesion following hematogenous seeding of bacteria into a structurally normal gland or may complicate preexisting adrenal hemorrhage. Infection with both Gram-negative and Gram-positive organisms has been reported. The presenting signs are pyrexia, irritability, abdominal distention, and vomiting.
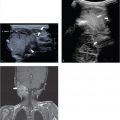
Prompt diagnosis is necessary. US can help to localize the source of the infection within the adrenal gland, where a complex mass containing debris-laden fluid and septa is observed ( Fig. 14.10 ).

The abscess(es) must be drained, and a percutaneous approach is preferred ( Fig. 14.11 ).

14.5.5 Congenital Adrenal Hyperplasia
Congenital adrenal hyperplasia is associated with a group of enzyme deficiencies, most inherited in an autosomal-recessive manner, in which normal steroid synthesis by the adrenal cortex is impaired. Deficiency of 21-hydroxylase is the most common cause of congenital adrenal hyperplasia, accounting for 90% of cases, and is confirmed by elevated serum levels of the precursor 17-hydroxyprogesterone. The adrenal cortex cannot synthesize cortisol efficiently, and this is associated with excessive production of ACTH by the pituitary gland. The result is overproduction of cortisol precursors, some of which are diverted to sex hormone biosynthesis. The excess androgens cause virilization, leading to ambiguous genitalia in female newborns and rapid postnatal growth in both sexes. A concomitant deficiency of aldosterone leads to the salt-losing crisis, in which infants present with hypovolemic shock and failure to thrive.
The adrenal glands in children with congenital adrenal hyperplasia may be enlarged. The adrenal gland may retain the typical y or v shape, and the internal architecture is preserved. An enlarged gland can be measured, according to Sivit et al, as an adrenal limb greater than 4 mm in width and greater than 20 mm in length. The cerebriform pattern is a characteristic finding whereby the wrinkled surface of the gland resembles the brain gyri and contrasts with the usually smooth surface of the neonatal gland ( Fig. 14.12 ).

Tips from the Pro
Sonography is useful to examine the pelvic organs in a virilized female neonate. The uterus may appear normal in outline but lacks an endometrial stripe.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree