6 Mediastinum
Ultrasound of the mediastinum is particularly useful to delineate anomalies of the mediastinum and great vessels, evaluate masses in children with mediastinal widening, search for lymph nodes, and assess malposition and complications of central vein catheters. It can also be used to perform biopsies. Ultrasound is quickly implemented in the remote intensive care situation in which patients can be examined in any given position and location, thus minimizing the need to move or transfer persons who are on life support devices.
To correlate findings, it is always recommended to review the patient’s most recent chest radiograph before the ultrasound examination. The ultrasound examination of the pediatric mediastinum is facilitated by the nonossified sternum and thymus, especially in newborns and infants.
To analyze mediastinal structures, a supraclavicular, suprasternal, trans-sternal, parasternal, subcostal, or subxiphoidal approach is used. In neonates and small infants, the sternum is predominantly cartilaginous and allows a trans-sternal approach. A supraclavicular or suprasternal approach is best performed with the patient’s shoulders lifted by a helping hand or by a pillow or rolled-up towel placed under the patient’s shoulders to extend the neck. Turning the patient’s head to the opposite side gives also free access to the region.
The transducer is placed above the sternum or clavicle and tilted posteriorly. This position offers a high degree of flexibility for documenting mediastinal structures in different planes.
Axial and sagittal parasternal views are acquired with the transducer positioned next to the sternum as the patient lies in the supine position.
For subcostal or subxiphoidal ultrasound access, the transducer position is immediately below the xiphoid process and along the lower border of the thoracic cage. Transverse, coronal, and sagittal scans image intrathoracic pathology along with the spine, inferior vena cava, and aorta and determine the situs.
Transducers are selected according to the size of the patient and the position of the lesion being evaluated. High-frequency linear and sector transducers are used for examining preterm infants, newborns, and small infants, and for evaluating the sonographic patterns of mediastinal masses. Small transducers are valuable to insonate from the supraclavicular or suprasternal notch. Changing the patient’s position will delineate the position dependency of a lesion and can help to move intestinal air out of sight. It may be helpful to give the patient something to drink during the examination to delineate the esophagus, and to fill the stomach with fluid, which then provides an excellent acoustic window.
The following paragraphs will describe how to reliably document normal vascular anatomy and will illustrate the most common mediastinal pathologies accessible to ultrasound.
6.1 Normal Anatomy and Variants
6.1.1 Thymus
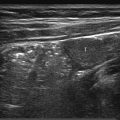
The thymus ( Fig. 6.1a) is located in the anterior superior mediastinum and changes in size, configuration, and position with the patient’s age ( Table 6.1 ). In newborns and infants, the normal thymus is visible in trans-sternal, parasternal, and suprasternal views. It is bilobed with smooth margins and has a homogeneous, fine, granular echotexture with some regular linear and punctate echogenicities that most likely represent connective tissue. On Doppler imaging, it has scant vascularity. It is an easily deformable organ that, even when large, does not compress or displace neighboring vascular structures. The thymus gland responds to acute physiologic stress with rapid and severe involution. After cessation of the stress, it takes weeks to months to regenerate and may then be enlarged because of “rebound growth.”



Thymic dimensions, cm (SD) | |||||
Age (months) | No. of cases | Transverse | AP right | AP left | Length |
Premature | 20 | 2.9 (0.3) | 1.5 (0.4) | 1.5 (0.4) | 3.1 (0.4) |
0–1 | 25 | 3.3 (0.3) | 1.6 (0.3) | 1.8 (0.3) | 3.6 (0.3) |
1–2 | 21 | 3.6 (0.6) | 1.9 (0.3) | 2.0 (0.3) | 3.9 (0.4) |
2–3 | 11 | 3.7 (0.5) | 1.9 (0.3) | 2.1 (0.3) | 3.8 (0.3) |
3–4 | 15 | 3.8 (0.4) | 1.9 (0.5) | 2.2 (0.6) | 4.0 (0.4) |
4–5 | 7 | 4.0 (0.6) | 2.0 (0.6) | 2.2 (0.4) | 4.1 (0.3) |
5–6 | 8 | 3.9 (0.2) | 1.9 (0.4) | 2.3 (0.3) | 4.0 (0.2) |
6–8 | 8 | 3.6 (0.5) | 1.8 (0.4) | 1.9 (0.5) | 3.6 (0.6) |
8–10 | 14 | 3.7 (0.5) | 1.7 (0.5) | 1.9 (0.5) | 3.7 (0.4) |
10–12 | 5 | 3.7 (0.5) | 1.9 (0.6) | 1.8 (0.5) | 3.6 (0.2) |
12–18 | 7 | 2.8 (0.5) | 1.2 (0.5) | 1.4 (0.4) | 3.2 (0.9) |
18–24 | 10 | 3.3 (0.4) | 1.2 (0.4) | 1.2 (0.3) | 3.4 (0.7) |
Abbreviations: AP, anteroposterior; SD, standard deviation. Source: Reproduced with permission of American Institute of Ultrasound in Medicine (AIUM) from Yekeler E, Tambag A, Tunaci A, et al. Analysis of the thymus in 151 healthy infants from 0 to 2 years of age. J Ultrasound Med 2004;23(10):1321–1326; permission conveyed through Copyright Clearance Center, Inc. Note: Mediastinal ultrasound was performed in 151 children (79 boys and 72 girls). All children were healthy and had no stress factors affecting thymic size. The maximum transverse diameter, right lobe AP length, and left lobe AP length were assessed. Perpendicular to the transverse plane, the longest craniocaudal length was assessed. | |||||
6.1.2 Trachea
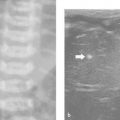
The trachea ( Fig. 6.1b) runs together with the esophagus in the midline, and both can be used to determine the midline of the mediastinum. In longitudinal section, the wall of the trachea has the appearance of a necklace because of its hypoechoic cartilages.
6.1.3 Esophagus
The esophagus ( Fig. 6.1c) is a muscular tube consisting of an echogenic mucosa and submucosa and a hypoechoic muscle wall. The movement of air and fluid in its lumen can be visualized by ultrasound imaging, and delineation of the esophagus can be improved when the patient swallows fluid.
6.1.4 Heart and Great Vessels
The aortic arch ( Fig. 6.1d) is scanned from a suprasternal, supraclavicular, or high parasternal transducer position ( Fig. 6.1e). A high transverse scan at the level of the manubrioclavicular junction demonstrates the aortic arch branches in cross-section: the larger innominate artery and the smaller left common carotid artery and left subclavian artery. The left brachiocephalic vein runs directly anterior ( Fig. 6.1f). Shifting the transducer to a lower level reveals the left-sided aortic arch passing from right to left anterolateral to the trachea and esophagus ( Fig. 6.1g). A transverse scan more caudally visualizes the right superior vena cava, ascending aorta, main pulmonary artery, and right pulmonary artery.
As the left pulmonary artery runs more cephalad than the right, a slight anticlockwise rotation of the transducer is needed to image the pulmonary artery bifurcation as a whole ( Fig. 6.1h). On a coronal scan from the suprasternal fossa, the left innominate vein, which joins the superior vena cava, runs above the aortic arch, which is cut in cross-section ( Fig. 6.1j). The right pulmonary artery runs beneath the aortic arch and behind the superior vena cava ( Fig. 6.1e, h). In a sagittal oblique plane, the ascending aorta, the aortic arch with its usually three brachiocephalic vessels, and the proximal descending aorta are shown in a single image. The right pulmonary artery is seen in cross-section below the arch ( Fig. 6.1e). To reliably determine the side of the aortic arch, the trachea or esophagus must be identified as a midline marker. Also, visualization of a right or left innominate artery makes it possible to determine the side of the aortic arch. A right innominate artery implies a left aortic, arch and vice versa. A plane directed from the suprasternal notch toward the right shoulder visualizes the innominate artery and its bifurcation into the right subclavian and carotid arteries ( Fig. 6.1i). In the sagittal right parasternal scan, the superior vena cava and azygos vein entering the posterior aspect of the superior vena cava are visualized. Subcostal transverse and sagittal planes serve to image the inferior vena cava, the entire thoracic aorta, and the spine. If present, azygos continuation of the inferior vena cava can be shown.
Tips from the Pro
The trachea and esophagus are important in locating the midline of the mediastinum. On a longitudinal scan, at least one of these two structures has to be identified to determine the side of the aortic arch. Failure to identify the trachea or esophagus may cause a misinterpretation of the position of the aortic arch in cases with midline shift to one side of the chest (e.g., hypoplasia or agenesis of a lung, atelectasis of lung parenchyma on the side of the shift, a contralateral mass).
6.2 Pathology
6.2.1 Thymus
Aberrant or Ectopic Thymus
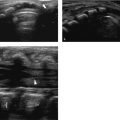
Congenital anomalies of the position of the thymus are classified as aberrant or ectopic. The normal pathway of embryonic thymic descent is from the angle of the mandible to the superior mediastinum.
Aberrant thymus is due to incomplete or missing descent, leading to remnants of thymic tissue positioned in any location along the normal pathway of descent. Most often, there is an incidental finding of a mass in the lateral neck or in the suprasternal area without signs of airway obstruction or compression of adjacent vessels. As in persons with a normal thymus, multiple linear echoes and discrete echogenic foci are found ( Fig. 6.2a, b).

By contrast, an ectopic thymus can be found in any position except the normal pathway of embryologic descent of the gland (i.e., pharynx, trachea, posterior neck or mediastinum, or esophagus). There is often continuity with the normally positioned thymus. When an ectopic thymus is located in the posterior mediastinum or within the trachea or pharynx, its visualization during an ultrasound examination can be obscured by overlying ribs or air. Awareness of this uncommon entity and knowledge of its variable presentation are essential to avoid unnecessary surgery.
Thymic Aplasia
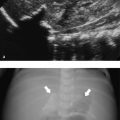
The thymus is responsible for the development of T-cell immunity. Thymic aplasia or hypoplasia is associated with T cell–related immunodeficiencies such as 22q11.2 deletion syndrome (cardiovascular [especially aortic arch] anomalies, abnormal facial features, thymic hypoplasia, cleft palate, hypocalcemia), ataxia telangiectasia, and severe combined immune deficiency syndrome (SCID). SCID syndrome is characterized by the absence of normal thymic tissue in all cases. Mediastinal ultrasound will document agenesis or hypoplasia of the thymus and associated cardiac and aortic arch anomalies ( Fig. 6.3a–c).

Tips from the Pro
Absence of the thymus can be an important feature in an infant presenting with recurrent or severe infections during the first months of life. The radiologist may be the first to suggest the diagnosis of an immunodeficiency syndrome ( Fig. 6.3a).
6.2.2 Trachea
Subglottic Hemangioma
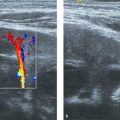
Infantile hemangioma is the most common vascular tumor of infancy. This benign neoplasm is characterized by a rapid endothelial proliferation stage followed by a slower involution stage. The lesions can be isolated or multiple anywhere in the body, most frequently in the skin and subcutaneous tissue.
Localization within the epithelial lining of the trachea is rare but may result in tracheal stenosis presenting with biphasic stridor. Symptoms develop frequently before 6 months of age. Progressive airway obstruction during the proliferative phase has the potential to be life-threatening. When large, the hemangioma infiltrates the soft tissue around the trachea or the thyroid gland. An association between the presence of cutaneous hemangioma in the beard distribution and airway involvement due to derivation from the same embryologic structures is known.
Ultrasound documents the characteristic features of hemangioma. There is a well circumscribed solid mass with variable echogenicity ( Fig. 6.4a, b). Draining and feeding vessels may be identified. Blood flow, documented with color Doppler, varies between exuberant and barely detectable ( Fig. 6.4c).

Tracheobronchial Calcification
Calcification of the cartilaginous rings of the trachea and bronchi ( Fig. 6.5a, b) is part of the natural process of aging. In children, it is rare and often associated with laryngeal calcification. It has been described in patients with chondrodysplasia punctata, Keutel syndrome, warfarin embryopathy, adrenogenital syndrome, or diastrophic dysplasia and (as in our patient) after long-term warfarin therapy.

6.2.3 Esophagus
Esophageal Atresia and Tracheoesophageal Fistula
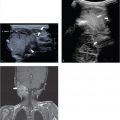
Esophageal atresia ( Fig. 6.6a–d) and tracheoesophageal fistula are the most common congenital malformations of the esophagus. The prognosis is influenced by the frequent associated anomalies such as congenital heart disease, gastrointestinal anomalies, and the VA(C)TER(L) complex (vertebral defects, anal atresia, tracheoesophageal fistula, radial aplasia, renal dysplasia, and cardiac and limb anomalies). In the majority of patients, the atresia is between the proximal and middle thirds of the esophagus and is associated with a distal fistula (Vogt type IIIb). Because the patients can swallow, isolated tracheoesophageal fistula may not be detected early.

Ultrasound technique allows a clear documentation of the length and condition of the wall of the proximal pouch and its relation to surrounding structures (i.e., aortic arch; Fig. 6.6a). Visualization may be enhanced by the instillation of saline solution into the pouch (Fig. 6.6 b, c). An isolated tracheoesophageal fistula as well as one or rarely multiple fistulas from the proximal pouch in esophageal atresia may be recognized by the presence of tiny air bubbles that move in the soft tissue between the trachea and the esophagus ( Fig. 6.6d, Fig. 6.7a–c).

Tips from the Pro
A determination of the side of the aortic arch by ultrasound is important before surgery, as thoracotomy will be on the opposite side of the aortic arch.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree