11 Portal and Mesenteric Venous Disorders
Summary
The array and complexity of portal vein interventions represent an area of challenge for the interventional radiologist. These procedures require an in-depth understanding of the relationship between hepatic, cardiac, and renal physiology, and may be intellectually and clinically satisfying when performed well. This chapter reviews the broad spectrum of portal venous interventions performed for a variety of clinical syndromes, providing technical guidance as well as clinical management tools for the beginning and advanced practitioner.
11.1 Introduction
Endovascular interventions in the portal venous system have become standard in current medical practice. These procedures range from elective procedures for the management of chronic problems to emergency procedures performed for patients who are in critical condition, such as patients with acute variceal hemorrhage. Interventions in the portal venous system may be technically challenging, laborious, and time consuming. The purpose of this chapter is to discuss the broad range of portal venous interventions as well as their planning.
11.2 Case Vignette
11.2.1 Patient Presentation
A 53-year-old man with a history of cirrhosis secondary to nonalcoholic steatohepatitis and refractory ascites requiring frequent large-volume paracentesis presented emergently with upper gastrointestinal bleeding. The patient had a preexisting occluded transjugular intrahepatic portosystemic shunt (TIPS), with cavernous transformation of the portal vein. Attempts to open his shunt at the outside hospital had been unsuccessful. On presentation, esophagogastroduodenoscopy was performed to treat an active area of bleeding from the esophageal varices, and gastric antral vascular ectasia was treated with epinephrine injection and clip placement. Attempts to sclerose the esophageal varices failed, and the interventional radiology team was consulted to evaluate the TIPS and attempt recanalization or new TIPS creation.
11.2.2 Physical Exam
The patient was critically ill, with jaundice and abdominal distension due to ascites. Laboratory values were as follows: prothrombin time/international normalized ratio (INR), 2.0; creatinine, 2.2 mg/dL; bilirubin, 2.1 mg/dL; alanine aminotransferase, 160 U/L; aspartate aminotransferase, 102 U/L; albumin, 2.5 g/dL; sodium level, 135 mmol/L; and platelets, 65 k/uL. The calculated corrected Model for End-Stage Liver Disease (MELD) score for the patient was 25 (estimated 3-month mortality: >50%).
11.2.3 Imaging
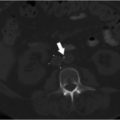
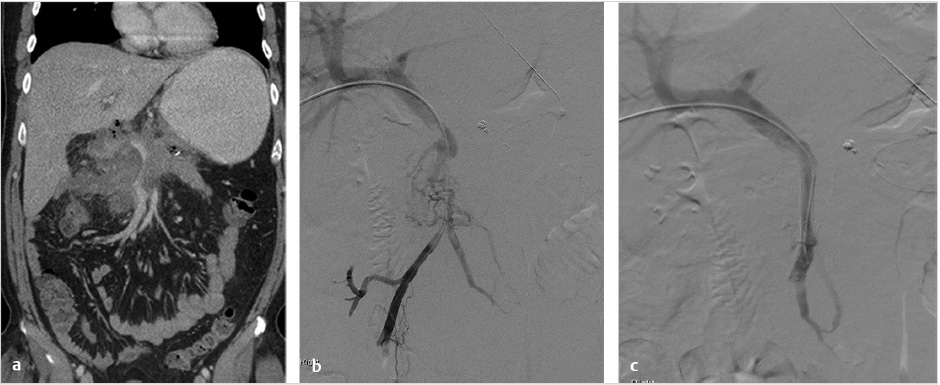
Contrast-enhanced CT of the abdomen was performed and demonstrated chronic portal vein thrombosis (PVT). The TIPS was occluded. There was nonocclusive thrombus in the superior aspect of the superior mesenteric vein (SMV). The splenic vein and left portal vein were patent. Multiple small collaterals were identified around the anticipated anatomic location of the right portal vein.
11.2.4 Specifics of Consent
The following risks were discussed with the patient prior to proceeding:
Procedure failure due to PVT or because of other technical difficulties.
Bleeding, either from capsular disruption or from fistula creation.
Liver failure due to portal venous shunting. In such cases, liver transplant must be considered as a therapeutic alternative.
Deterioration of renal function. A mild elevation in creatinine level increases the risk, and this risk is increased in patients with acute anemia secondary to bleeding. This risk was discussed with the patient.
Infection of the TIPS stent graft. In cases of spontaneous bacterial peritonitis or other known infection, a bare metal stent can be used in place of a covered stent.
The patient’s MELD score was 25, a high-risk patient. The clinical predictive value of MELD decreases in the emergent setting, but risk of mortality should be discussed with the patient.
PVT increases the difficulty of TIPS placement, so preprocedural planning was extremely important.
11.2.5 Details of Procedure
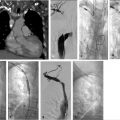
The procedure performed was a TIPS with portal vein recanalization. The procedure was performed under general anesthesia. Recanalization of the preexisting TIPS was not attempted as it was thought it would be technically difficult and potentially not useful. The procedure was performed with simultaneous transjugular and transarterial approaches. The most important role for the transarterial approach was to perform a superior mesenteric artery (SMA) injection to identify the largest collaterals suitable for transhepatic puncture. Once the most suitable collaterals were identified, a transhepatic puncture was performed, aiming to the largest suitable collateral. After a successful entry into a large collateral, guidewire and catheter manipulation to negotiate the collateral was performed until a large branch of the SMV was entered. An extended shunt was created from the superior mesenteric branch to the right hepatic vein. The most distal aspect of the shunt was created with a noncovered stent and the transhepatic tract was treated with a VIATORR stent graft (W.L. Gore & Associates, Flagstaff, AZ).
11.2.6 Follow-up
Liver function tests are critical to identify immediate changes after TIPS creation. Hemodynamic changes after TIPS are known to affect liver function and the interventionalist should be attentive to those changes. Assessment for postprocedural bleeding is also critical at this stage.
Early and frequent assessment of mental status is recommended to identify signs of hepatic encephalopathy. A TIPS reduction may be necessary if encephalopathy is refractory to medical management. This event occurs in 3 to 7% of cases.
A vascular ultrasound of the liver should be obtained approximately 4 to 5 days postprocedure to assess shunt patency, and repeated every 6 months for early identification of stenosis or thrombosis.
11.3 Portal Hypertension
The liver receives its blood supply predominantly via the portal venous circulation, which accounts for 75 to 80% of the blood flow; the remaining 25% is supplied by the hepatic artery. The portal circulation is a low-pressure system with minimal resistance in which blood traverses the portal triad and the sinusoids before draining into the hepatic veins. The portal vein flow (PVF) follows Ohm’s law, where the portal vein pressure (PVP) is the product of the PVF and the intrahepatic venous resistance. 1
Portal hypertension (PH) occurs as the result of increased resistance to PVF in conditions such as cirrhosis, intra- and extrahepatic tumors, and outflow obstructions such as Budd–Chiari syndrome. Due to these conditions, the PVF directed toward the liver (hepatopetal) is reduced, or may be forced retrograde (hepatofugal). This situation results in a constellation of clinical signs and symptoms caused by increased blood volume in the splanchnic circulation, which induces the development of collateral veins and body fluid redistribution. 2 The major consequences are the development of extensive varices in the gastrointestinal tract that can cause severe or even fatal episodes of bleeding, and refractory ascites or hydrothorax with multiple hemodynamic implications.
Endovascular treatments are typically used as a rescue therapy in patients with PH and variceal gastrointestinal bleeding or refractory ascites who have not responded to more conservative medical treatments. 3 In such cases, endovascular therapies are used to decompress the portal venous system and reduce the PVP, thereby redistributing and redirecting the splanchnic flow. 3
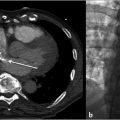
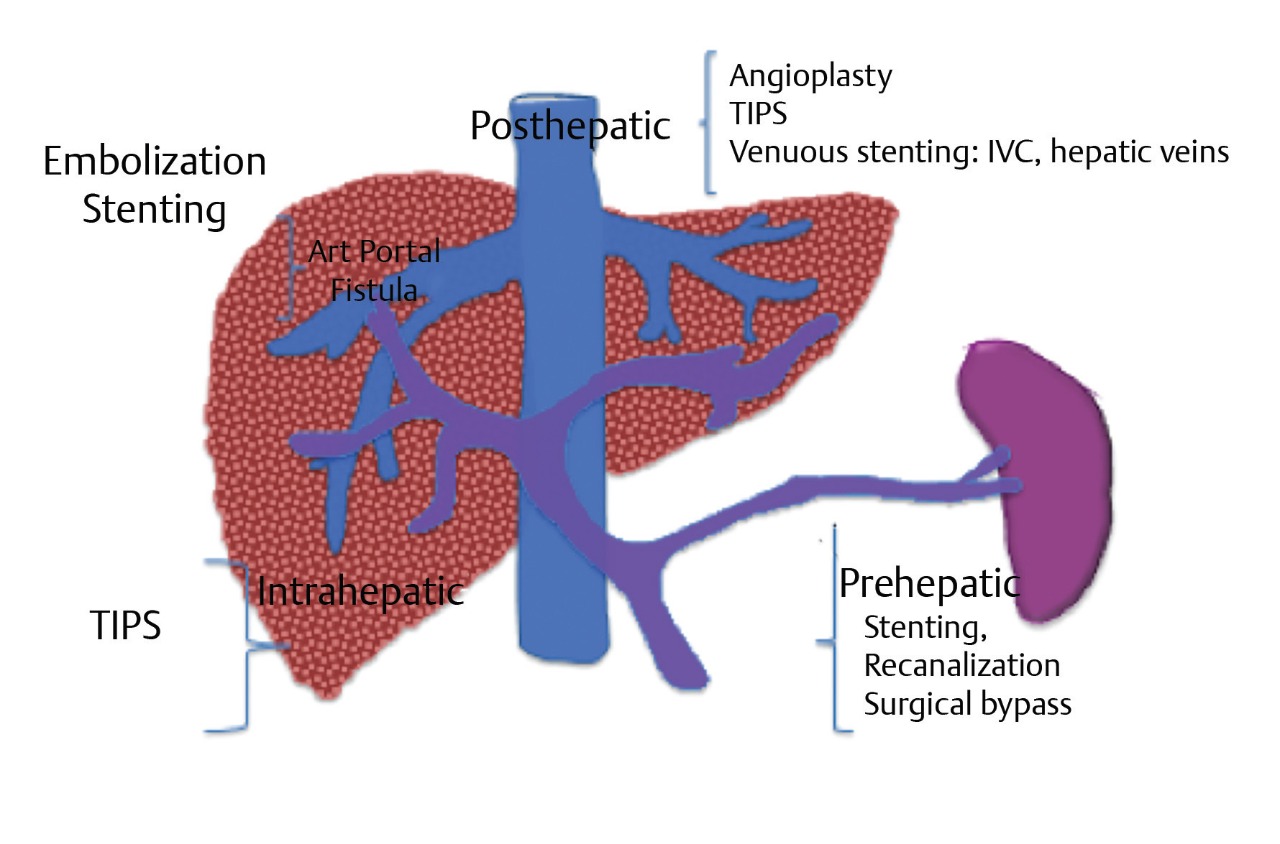
PH is classified as prehepatic, intrahepatic, or posthepatic based on anatomic levels (Table 11.1; Fig. 11.1). The etiology of PH and its anatomic correlation are used to determine which endovascular therapeutic approach is optimal. The therapeutic options include: portal vein recanalization with angioplasty and stenting in cases of prehepatic PH (Fig. 11.2 ; Fig. 11.3); hepatic vein or inferior vena cava (IVC) interventions in posthepatic PH; and TIPS creation in cases of intrahepatic and posthepatic PH.
11.3.1 Patient Presentation and Evaluation
The hepatic venous pressure gradient (HVPG) measures PVP obtained during hepatic vein catheterization. The HVPG measures the gradient between the higher portal venous pressure and the lower hepatic vein pressure; it is this gradient that drives portal blood through the sinusoids and into the hepatic veins.
To calculate the HVPG, the free hepatic vein pressure should be measured approximately 4 cm upstream to the confluence into the IVC, and the wedge pressure should be measured with balloon inflation in the same anatomic region within the hepatic vein. Balloon occlusion should be confirmed by visualizing sinusoidal opacification during contrast injection. In addition to sinusoidal contrast, contrast should be noted filling the portal veins in retrograde fashion. If hepatic vein to hepatic vein collaterals are noted during balloon occlusion injection, the subsequent pressure gradient measurements will be indeterminate since the balloon occlusion measurement will actually be of the collateralized hepatic vein rather than of the portal venous system.
The HVPG corresponds to the difference between the wedge hepatic venous pressure, which is equivalent to the portal venous pressure, and the free hepatic venous pressure, which is similar to the IVC pressure.
HVPG = wedge hepatic pressure – free hepatic pressure
In humans, normal HVPG values range from 1 to 5 mm Hg; an HVPG value > 12 mm Hg represents clinically significant PH. 3 , 4 Additionally, HVPG can be used as a measure of success of PH treatment, which is typically defined as a decrease in HVPG of >20% compared to preintervention values, or a posttreatment HVPG value <12 mm Hg. 3
11.4 Transjugular Intrahepatic Portosystemic Shunt
11.4.1 Indications for Transjugular Intrahepatic Portosystemic Shunt
The TIPS procedure creates an endovascular, transhepatic shunt that decreases the HVPG. If successfully created, the bypass across the liver decompresses the high pressure mesenteric, splenic, and portal venous system, diverting flow via the stent directly into the systemic circulation via the hepatic veins and right atrium (low-resistance outflow pathway). 3 The procedure is most frequently used to relieve variceal bleeding and ascites refractory to medical treatment. First-line therapy for bleeding varices typically includes nonselective beta-blockers and endoscopic band ligation. Currently, TIPS are usually placed when endoscopic and medical therapies are not successful. 4
In treating variceal bleeding, TIPS decreases HVPG by directly diverting flow into the systemic circulation. In refractory ascites, TIPS works by increasing natriuresis by reducing proximal tubular sodium resorption in the renin–angiotensin–aldosterone axis. TIPS is more effective in controlling recurrent or refractory ascites when compared to large-volume paracentesis; however, encephalopathy rates are higher in patients undergoing TIPS. Additionally, a significant benefit on survival has not been demonstrated. 5
In addition to the common indications of variceal bleeding and refractory ascites, TIPS can be used in patients with PH who are to undergo major abdominal surgeries (e.g., hernia repair, colectomy, cholecystectomy). By decompressing the portal system and varices, TIPS is used to prevent intraoperative bleeding. In patients with Budd–Chiari syndrome, TIPS can be used in addition to or after the failure of other interventions, such as hepatic venous thrombectomy, angioplasty, and/or stenting of the hepatic vein outflow. 6
Transjugular intrahepatic portal vein shunting can also provide safe portal venous access in patients with ascites or peritoneal lesions when a percutaneous transhepatic approach cannot be used. Such indications may include cases in which SMV interventions are required (e.g., SMV thrombosis, stenosis), in cases of posttransplant interventions, or in the operative scenario of pancreatic cell transplantation/infusion into the portal vein.
Some clinicians use TIPS as a bridge to liver transplant in patients with end-stage liver disease; this indication is typically reserved for patients with clinically significant sequelae of their PH (e.g., varices at risk for bleeding). After orthotopic liver transplantation, TIPS can also be used to treat complications of PH, such as Budd–Chiari syndrome, PVT, and recurrent PH. 7 TIPS has also been used in the treatment of hepatic hydrothorax and hepatorenal syndrome; however, its use in these conditions remains controversial. 5 , 8 The indications and contraindications for TIPS procedures are described in Table 11.2.
Indications for TIPS procedure |
|
|
|
|
|
|
|
|
Contraindications for TIPS procedure |
Absolute
|
Relative
|
11.4.2 Preparation for Procedure
Careful patient evaluation is essential before proceeding with TIPS. Patient and family members must have a clear understanding of the risks and potential benefits of the procedure. In critical situations such as massive, acute variceal bleeding, TIPS is performed as a life-saving procedure, and a full patient evaluation may not be feasible. 9 It has been demonstrated that emergent TIPS procedures are associated with a higher mortality rate than those performed electively. 10 , 11
Prior to performing the procedure, it is important to obtain a complete history from the patient and evaluate the symptoms. Particular attention should be paid to preexisting hemodynamic and respiratory conditions. Although right heart catheterization and pressure measurements will be obtained during the TIPS procedure, a preintervention echocardiogram is recommended. On echocardiography, the presence of heart failure, abnormal right ventricular function, pulmonary artery hypertension (pulmonary wedge pressure > 45 mm Hg), and tricuspid regurgitation constitute absolute contraindications to TIPS. In addition, the presence of a significant right-to-left shunt can increase perioperative morbidity 10 ; an echocardiographic bubble study with vascular ultrasound is recommended to assess whether the risk of paradoxical embolus will be significant (e.g., in cases of acute portal or hepatic vein thrombosis). Other risk factors to assess for during the initial clinical evaluation include increased age, preexisting encephalopathy, elevated liver enzymes, and the presence of ascites.
In current clinical practice, the MELD score is used for pre-TIPS risk stratification. This score is the most accepted predictor for post-TIPS short- and intermediate-term mortality and post-TIPS outcomes. 3 , 12 The variables used in calculating the MELD score are serum total bilirubin, INR, and serum creatinine level. With this score, TIPS procedures are classified as low risk (MELD score ≤ 18), intermediate risk (MELD score 19–25), or high risk (MELD score >25), with corresponding 3-month mortality rates of 15, 33, and 80%, respectively. 3 , 13
The most recent laboratory values, including complete serum electrolytes, complete blood count, liver and kidney function panel, coagulation tests including INR, and blood type matching, in case blood products are required, should be obtained. In the context of suspected infection, the recommendation is to obtain prior blood and body fluid cultures. If current infection exists, the risk of performing the procedure versus waiting should be determined. Even in the setting of no ongoing infection, prophylaxis with wide-spectrum antibiotics before the TIPS procedure is recommended routinely. 14
Paracentesis may be performed in the days prior to the procedure to reduce ascites volume, and should be repeated immediately before the procedure to reduce the risk of bleeding, hypotension, and dehydration. 5 Infusion of intravenous concentrated albumin may assist with intravascular volume retention when large volumes of ascitic or pleural fluid are drained; this infusion may also help prevent contrast-induced nephropathy or worsening prerenal failure. Exceptional situations include cases of large-volume pleural effusion or ascites in the perioperative period that limit adequate ventilation or preclude prompt postoperative extubation. In these settings, more aggressive preoperative fluid removal may be indicated.
The decision to use general anesthesia versus moderate sedation should be based on the mental, hemodynamic, and respiratory status of the patient, the risk of aspiration, the estimated time required for the intervention, and the preference of the operating physician.
Pre-TIPS Imaging Evaluation
A recent liver vascular ultrasound and cross-sectional imaging of the abdomen with or without contrast are useful tools for assessing the portal and hepatic venous anatomy as well as the presence of venous collaterals and spontaneous portosystemic shunts. Imaging can also be used to exclude or confirm the presence of focal liver lesions that may interfere with intra- or transhepatic access. The operating interventional radiologist should also evaluate the patency of the hepatic artery and celiac trunk on these imaging studies. A significant arterial stenosis is considered a relative contraindication for TIPS since the hepatic arterial flow compensates for the liver blood flow that is shunted from the portal system.
Mapping the Portal Vein Target
The proposed intrahepatic pathway may be planned to use anatomic details obtained from a 3D multiplanar reconstruction (MPR) to evaluate the spatial relationship between the hepatic and portal veins (Fig. 11.4). The trajectory of the access is traced from the hepatic vein to the chosen intrahepatic portal venous branch. The preferred exit site in the hepatic vein should be approximately 1 to 2 cm from the IVC confluence to avoid difficult angles when the sheath is advanced across the liver parenchyma.
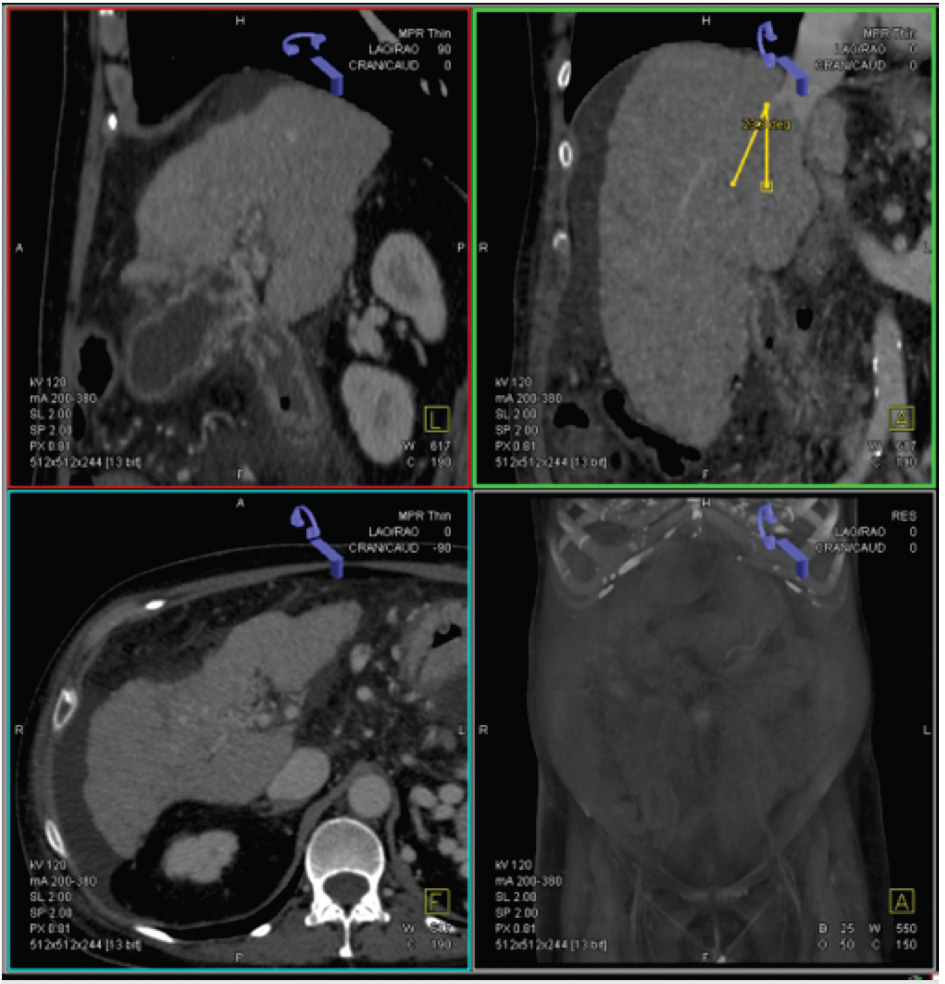
The orientation of the entry needle can also be determined depending on which hepatic vein is chosen. For instance, from the right hepatic vein, the needle must be directed ventrally in order to obtain access into the right portal vein. From the middle hepatic vein, the needle can be oriented dorsally if the puncture is made far from the portal bifurcation but should be oriented anteriorly if the puncture is very close to the bifurcation. From the left hepatic vein, the needle should be oriented ventrally and medially (Fig. 11.5).
All useful landmarks, including ribs, gallstones, and surgical clips, can be used as references. Those landmarks that are within the abdominal cavity are preferred over those outside the abdomen (e.g., bones), since the former will move with the liver during respiration. Obtaining preprocedure CT or MRI is useful if fusion software is to be used during the TIPS procedure. With this technology, across-sectional image can be used to reconstruct the vascular anatomy and bony landmarks, and the image can be fused with an image obtained by cone-beam acquisition of the upper abdomen during the TIPS procedure. The advantage of using such fusion technology is that this overlapped 3D image also rotates with fluoroscopy used in real time (Fig. 11.6).
Technical Tips and Tricks and Procedural Details
Once access is obtained in the jugular vein, a 10-French-long vascular sheath is advanced into the right atrium, and the central venous pressures (including the right atrial, right ventricle, and pulmonary venous pressures) are measured. If elevation of the pressures is demonstrated (e.g., >15 mm Hg), a wedge pulmonary artery pressure should be obtained to determine the degree of pulmonary hypertension.
The standard and most frequent approach to creating a TIPS involves right hepatic to right portal vein access; this is considered the safest way to avoid puncture of the hepatic artery, extrahepatic main portal vein, or biliary ducts. A left hepatic to left portal vein access has been correlated with a lower risk for encephalopathy development, but the reasons for this are not clearly understood. 15 Even with this advantage, a right hepatic vein to right portal vein approach is preferred due to favorable anatomic relationships.
In cases of challenging access to the hepatic vein, one may attempt transcaval access, parenchymal access via hepatic venous stumps, or direct intrahepatic portocaval shunt (DIPS) creation using fluoroscopy or intravascular ultrasound (discussed later in this chapter). 16 Strategies for accessing the hepatic veins are shown in Table 11.3.
Once in the hepatic vein, a wedge portogram, sometimes with improved visualization using carbon dioxide instead of iodinated contrast, viewed in at least two different obliquities should be obtained at an acquisition rate of at least four frames/second and set as a reference. By obtaining at least two obliquities, the anatomic relationship between the hepatic veins and portal veins can better be determined.
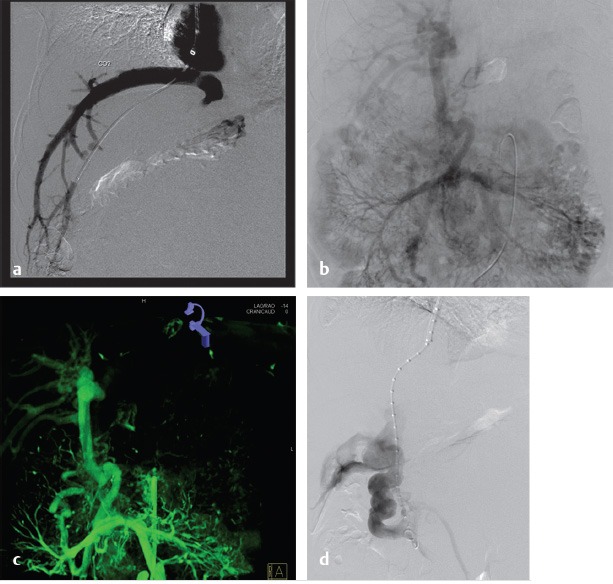
Conventional alternatives to acquiring a portogram for portal venous mapping include SMA angiogram with delayed venous portal phase and percutaneous access in a patent umbilical vein used in order to advance the catheter into the left portal vein (Fig. 11.7). If access into the portal vein is challenging despite the use of multiple techniques, a percutaneous access into a varix (Fig. 11.8) or transhepatic approach into the portal vein, with combined transjugular access in the hepatic vein, should facilitate access by providing a direct target for portal venous puncture.
After the TIPS needle is passed across the liver, gentle pull-back aspiration is performed until significant blood return is obtained. This should be followed by portal venogram to confirm the position of the needle. If access is deemed suitable for eventual TIPS placement, a hydrophilic wire in combination with a catheter should be negotiated distally followed by exchange for a stiff wire; catheter access is then obtained over the stiff wire. Once catheter access into the portal vein has been obtained, it is essential to perform a portogram to confirm that there is no contrast extravasation at the portal vein entry site, and measure direct portal venous pressures to determine the gradient. If the access is deemed appropriate, and the gradient and anatomy support the decision to proceed, the tract should be predilated with a 6- to 8-mm-diameter angioplasty balloon, with progressive advancement of the sheath immediately after or during balloon deflation until the portal vein is accessed with the sheath. If a severe angulation in the entry site to the portal vein is present, multiple maneuvers may be performed to advance the vascular sheath into the portal vein (Table 11.4). Extrahepatic access in the portal vein should be carefully evaluated and aborted if the operator determines that there is the high risk of peritoneal bleeding. By using a covered stent, extravasation around the portal vein entry site can be successfully treated by placing the graft segment across the vascular injury.
Direct PVP is obtained to establish a baseline portosystemic gradient (PSG) and to determine the appropriate diameter of the stent as well as the diameter of the angioplasty balloon. A PSG < 12 mm Hg may be misleading and raises the question of the presence of a potential unidentified spontaneous portosystemic shunt. In this scenario, it is very important to identify the spontaneous shunt on prior studies (CT or MRI) or in delayed venous phase SMA angiography. If the spontaneous shunt is not visualized during the maneuvers or during direct contrast portography, the decision to proceed with TIPS is based on whether signs and symptoms related to PH will be improved with TIPS placement.
Before stent placement, a portal venogram with a calibrated pigtail catheter is performed in order to plan the length of the stent and to determine where to deploy the covered and uncovered portions of the stent. If calibrated pigtail catheters are not available, the measurement may possibly be obtained directly by measurements from the angiographic software.
The VIATORR stent graft is often used during TIPS procedures due to its long history with such procedures, ease of use, and unique design combining covered and noncovered stent designs. The VIATORR is deployed with the uncovered portion advanced into the portal vein or right or left branches, and the downstream end deployed across the hepatic vein to the level of the hepatic vein–IVC confluence (Table 11.5).
Bare metal stents are still used in multiple institutions and, due to not being covered, are effective in cases of emergent TIPS when infection is suspected. Since 2004, when polytetrafluoroethylene (PTFE)-covered stent grafts were first approved by the U.S. Food and Drug Administration (FDA), multiple studies have shown decreased shunt dysfunction rates, increased long-term patency, decreased transmural permeation, and improved clinical outcomes with PTFE-covered stents compared to bare metal stents. 17 , 18 , 19
11.5 Hemodynamic Changes after TIPS Procedures
In the short term, the increased volume shunted to the heart through a TIPS can cause pressure elevation beyond the normal reserve of the right and left ventricle, elevating the pulmonary arterial pressure. Kovács et al 20 reported persistent transient post-TIPS enlargement of the left and right atria on cardiac MRI with normalization of cardiac dimensions 3 to 6 months after TIPS placement. Filì et al suggested that the volume expansion and hemodynamics following TIPS, including elevation of the cardiopulmonary pressures in patients with refractory ascites, returned to baseline at 24 hours postintervention 21 ; however, the number of patients in this study was limited. Colombato et al reported that after the increased volume load post-TIPS, a compensatory vasodilatory effect from splanchnic vessels decreases the peripheral and pulmonary circulation resistance. This mechanism helps explain the progressive hemodynamic adaptation noted after TIPS placement. 22
11.5.1 Combined TIPS and Variceal Embolization
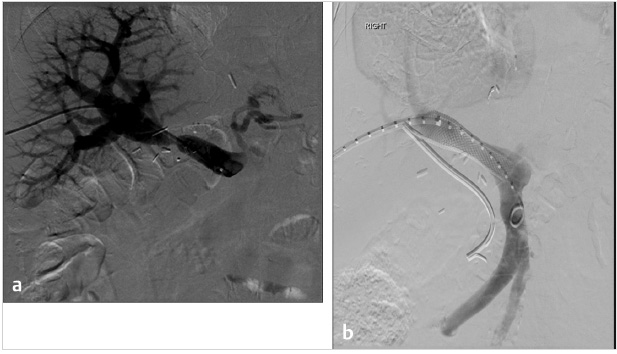
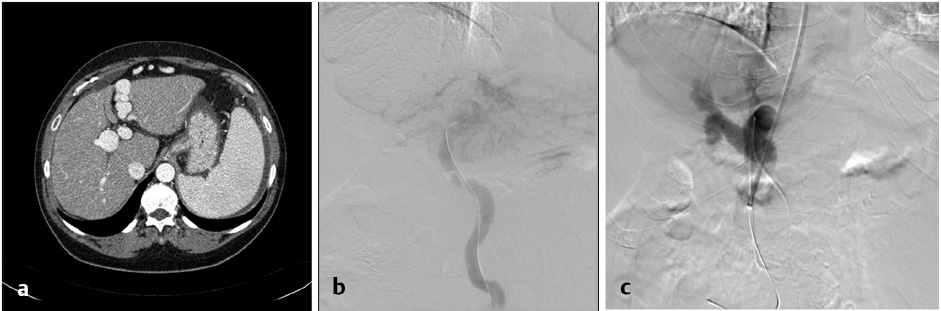
There are differing opinions regarding the necessity for variceal embolization following TIPS. Some operators chose to evaluate patients angiographically following TIPS placement to evaluate whether varices have resolved with the performance of shunting and forego embolization if there is no opacification of the varices (Fig. 11.9). Several studies have suggested that TIPS in combination with variceal embolization decreases the risk of recurrent variceal bleeding. Chen et al compared 106 patients with TIPS, including patients with and without variceal embolization, and concluded that the combination of TIPS and embolization decreased the risk of recurrent bleeding during the first few months post-TIPS. 23
Additionally, a recent meta-analysis suggested the benefit of performing variceal embolization during the TIPS procedure versus performing the TIPS procedure alone in the treatment of patients with variceal bleeding. 18 Adding variceal embolization at the time of the TIPS was found to reduce the incidence of variceal rebleeding (Fig. 11.10). Additionally, distal embolization of the varices using liquid embolic agents in combination with coils may help with long-term durability of embolization, preventing the possibility of recanalization. In contrast, a separate study concluded that the combination of TIPS with embolization was not beneficial in patients with a PSG < 12 mm Hg. 24 Some authors recommend performing variceal embolization before the TIPS procedure to prevent systemic migration of the embolic material. 19 The long-term outcomes of this technique are still unclear, as the variability in available embolization techniques and materials makes it difficult to compare study results.
11.5.2 TIPS and Chronic PVT
Cavernous transformation of the portal vein (cavernoma) acts as a compensatory mechanism after PVT. In this condition, multiple venous collaterals develop to supply and bypass the occluded portal vein. During this process, the native portal vein regresses, eventually degenerating into a fibrotic cord. 25 If the collateral pathways are not sufficiently large, PH may develop. Several classifications have been proposed for chronic PVT (Table 11.6), but predictors for successful recanalization of the vein have not been clearly defined. 26
|
|
|
|
Multiple techniques have been described for the treatment of PH caused by chronic PVT, including a conventional TIPS procedure, transhepatic or transsplenic portal recanalization, and DIPS placement. 27 TIPS can be very difficult if the portal vein has been transformed to a fibrotic cord. 28
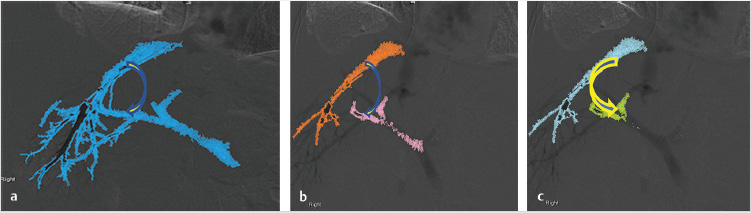
When a TIPS procedure is performed in the setting of cavernous transformation, the operator must first determine the feasibility of gaining access into a high-flow venous collateral, and must then determine where to insert the stent. The stent must be inserted in a collateral that will sufficiently decompress the varices and mesenteric circulation, while ensuing enough flow to keep the shunt patent. Prior to the procedure, cross-sectional imaging should be performed to determine the potential target vein.
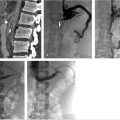
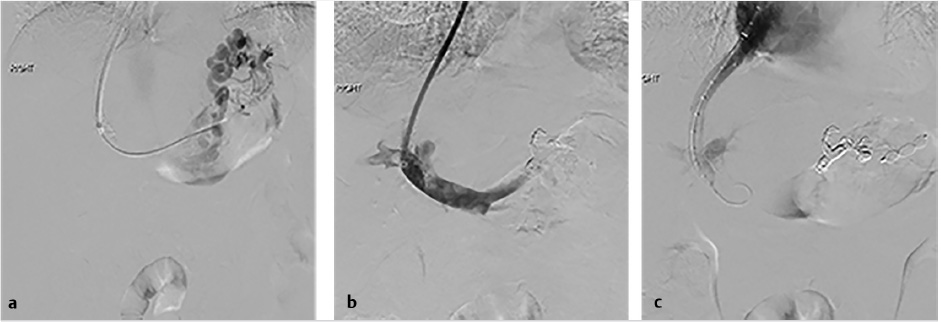
The initial part of the TIPS procedure for chronic PVT is identical to that of conventional TIPS procedures. A wedge hepatic portogram is used to establish a reference from at least two different angles. An SMA or splenic artery angiographic study can be used to evaluate whether an appropriate collateral vessel is present, and to determine where to place the stent (Fig. 11.11). The angiogram can be combined with cone-beam acquisition in a delayed venous phase to trace the proposed needle course and to identify the potential target vein. After evaluation of the imaging, a suitable collateral for access for TIPS placement can be selected (Table 11‑7)
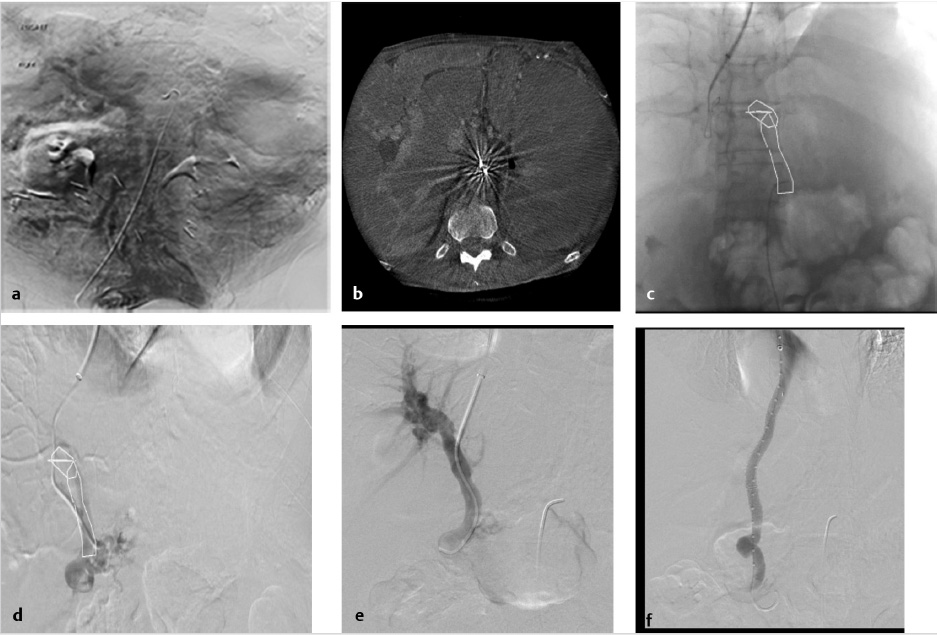
A transhepatic approach–assisted TIPS can be performed via percutaneous puncture of an intrahepatic portal vein, if there are no contraindications (e.g., severe ascites or varices around the access) to the procedure. In the case of severe ascites, a paracentesis should be performed immediately prior to percutaneous hepatic access. Once access into a portal branch is gained with a 21-gauge Chiba needle, a microwire can be directed centrally. At the authors’ institution, a 6-French Neff introducer (Cook Medical, Bloomington, IN) is inserted, and using a 4-French Glidecath (Terumo Interventional Systems, Somerset, NJ), access is obtained in the main portal vein. At this point, a direct portogram should be acquired to establish a target venous branch for the TIPS placement. In some cases, operators have inserted a low-profile angioplasty balloon into the thrombosed portal vein and inflated the balloon with contrast to highlight the target for the TIPS needle approach. 29
Transsplenic access involves a direct parenchymal approach through the spleen using a contrast injection to opacify the splenic vein and to advance a catheter retrograde into the portomesenteric vein confluence. Once access into the portal vein is achieved, a 10-mm gooseneck snare is placed within the peripheral intrahepatic portal branch to be targeted by the TIPS needle. Once the needle is place through the snare, an exchange length wire can be advanced from the jugular vein to the splenic access (through and through access), followed by tract dilation in the liver parenchyma and VIATORR stent deployment in normal fashion. Some operators perform embolization of the splenic access tract using coils or Amplatzer plugs, although some authors choose to use manual compression only. 30
The “gunsight technique” uses two gooseneck snares, one in the portal branch that is placed via the percutaneous approach, and one in the hepatic vein that is placed via transjugular access. The snares are crossed with a 21-gauge Chiba needle, and a microwire is inserted. The wire is manipulated through the jugular access to advance a catheter into the portal vein. 26 Once access is obtained in a portal venous collateral, manipulation of a 4-French catheter and hydrophilic wire can be performed until the splenomesenteric confluence is reached. In certain circumstances, a 0.018-inch microcatheter may be required to negotiate the vein.
A stent graft is generally recommended for deployment in the collateral vessel 31 ; however, a bare metal stent can also be used, specifically when the main collateral vessel receives inflow from other collaterals.
11.6 Potential Complications or Pitfalls
Capsular perforation is one of the main complications that can cause hemodynamic instability during or after TIPS procedures. This perforation can occur secondary to a laceration during a forced wedge hepatic venogram, or as an accidental extracapsular puncture with the TIPS needle.
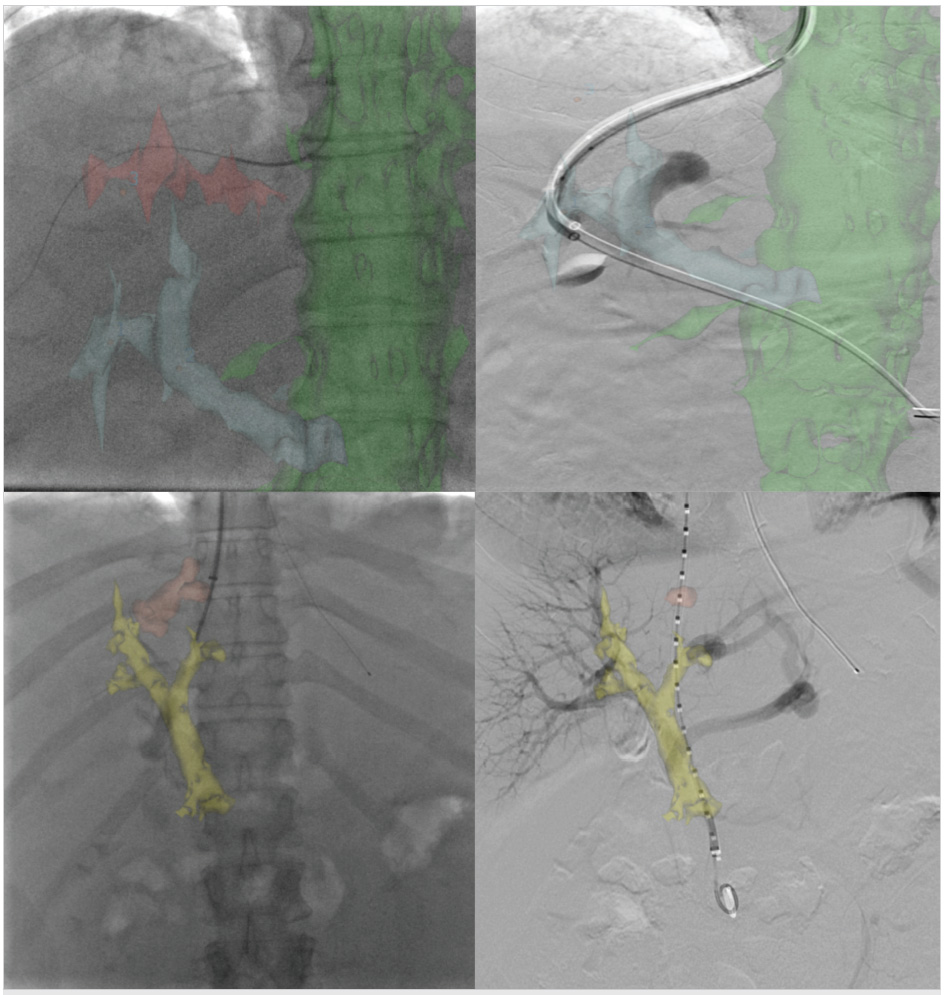
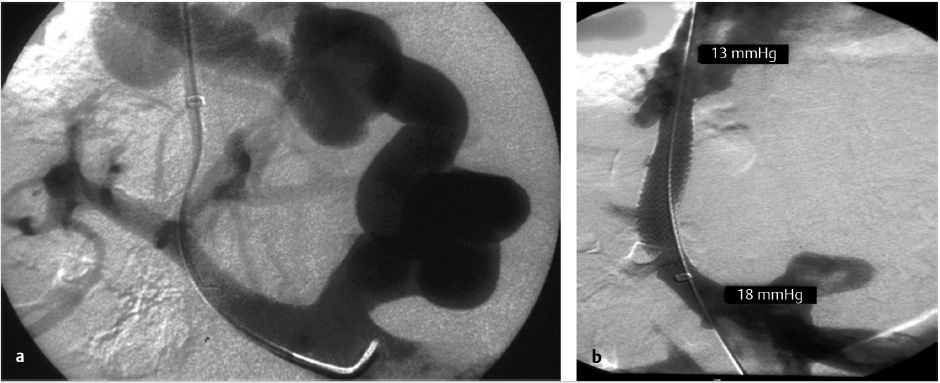
Portal venous bleeding, especially when an extrahepatic puncture leading to intraperitoneal bleeding is the source, must be treated immediately by the deployment of a covered stent graft (Fig. 11.12). If perforation occurs from wedge venography, contrast is injected through the catheter to localize extravasation in the vein. If the bleeding site is visualized, transcatheter embolization using coils can be performed (with a diameter upsize of at least 50% of the vessel diameter), or balloon-assisted Gelfoam injection to avoid the possibility of systemic migration. 32
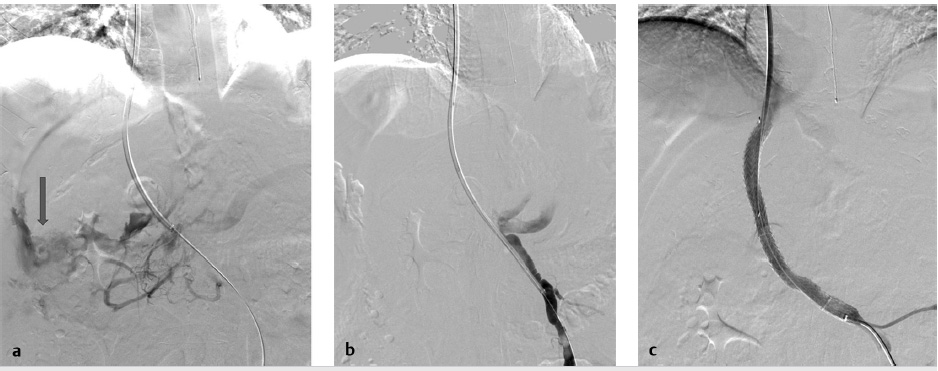
Most frequently, extracapsular punctures are clinically silent or are sufficiently treated with decompression of the portal system following stent deployment. However, in the setting of persistent hemodynamic compromise from an extracapsular puncture without clear contrast extravasation on venography, an arterial source of bleeding must be excluded by hepatic angiogram and, if required, transcatheter embolization performed (Fig. 11.13).
The clinical presentation of hepatic arterial puncture in a patient undergoing TIPS can range from irrelevant (no hemodynamic changes) to severe hemorrhage, hemobilia, arterioportal fistula (APF), and liver infarction with subsequent liver failure. If a catheter is positioned across the hepatic artery during percutaneous transhepatic access, gentle pullback injection with Gelfoam slurry is recommended to embolize the tract. If the main hepatic artery requires embolization, the surgical or transplant team should be consulted.
Stent migration retrograde into the main portal vein and the SMV confluence can be problematic for the potential anastomosis when the patient is a liver transplant candidate. In this situation, a second overlapping stent must be deployed to secure the migrated stent and prevent further retrograde movement. If the potential migration will be toward the right atrium, wire access should be maintained and the stent should be removed with a snare to prevent migration into the right ventricle or pulmonary arteries. 33
The most important adverse consequence after TIPS is hepatic encephalopathy, which can be seen in approximately 30% of cases. Hepatic encephalopathy has a direct effect on overall mortality following nonemergent TIPS procedures. 34 , 35 Clinically, encephalopathy is evaluated with psychometric tests, electroencephalogram, evoked potentials, and critical flicker frequency. The presentation of this condition ranges from minimal, in which mild cognitive impairment is present, to overt, which is associated with long-term sequelae of coma and death. 34 , 36 Recent studies have suggested that advanced age, the occurrence of encephalopathy before the TIPS procedure, and decreased liver function (assessed with Child–Pugh score) are the most important risk factors predicting the post-TIPS encephalopathy. 35 The most widely accepted pathophysiologic explanation for the occurrence of this condition is increased shunting of ammonia products from the splanchnic circulation. Ammonia and other splanchnic venous components behave as neurotoxins, and are normally cleared through hepatic metabolism. Additionally, some medications have been postulated to cause hepatic encephalopathy, including flumazenil and bromocriptine, through binding of gamma aminobutyric acid receptors. 35 , 37
Hepatic encephalopathy can be prevented by not overshunting the portal circulation. This means that the desired HVPG gradient after TIPS must be carefully measured and correlated with the MELD score and clinical condition to avoid overdilation of the stent. The presence of a concomitant spontaneous portosystemic shunt can aggravate encephalopathy by increasing the amount of ammonia products going into the systemic circulation and brain. The optimum post-TIPS HVPG depends on the indication for TIPS; the gradient expected to decrease recurrent bleeding should be reduced to <12 mm Hg, 38 , 39 and to decrease recurrent ascites, the gradient should be reduced to <8 mm Hg. If the gradient drops to <5 mm Hg, the risks of overshunting increase and therefore the risk of hepatic encephalopathy is elevated. However, Gaba et al reported self-expansion of TIPS stents over time and questioned the advantages of intraprocedural underdilation. 37 Initial therapy for encephalopathy is based on restricting dietary proteins, and increasing the clearance and decreasing the production of ammonia with nonabsorbable disaccharides such as lactulose. Lactulose acidifies the gut and inhibits the ammoniagenic enzyme (urease), decreasing ammonia production.
In addition, antibiotics such as rifaximin inhibit urease-producing bacteria and decrease ammonia production. 35 When medical therapy fails and significant encephalopathy persists, reduction of the TIPS should be considered (described below).
11.6.1 TIPS Reduction versus Occlusion
Interventional radiologists use a variety of techniques to reduce the TIPS diameter for patients with inadequate response to medical treatment for encephalopathy. Endovascular procedures are used to reduce the stent diameter or completely occlude the shunt to improve hepatopetal portal flow into the liver, indirectly decreasing the availability of the ammonia derivatives in the blood stream and blood–brain barrier. Because of the theory that TIPS outflow occlusion can cause a severe decrease in cardiac output, leading to sudden life-threatening shock, 37 careful evaluation using a balloon-occlusion test in the shunt is recommended before permanent occlusion is performed. With this technique, a baseline measure of the central venous pressure is obtained before an occluding balloon is temporarily inflated at the stent level. Immediately after temporary occlusion with the balloon, central venous pressure is remeasured to evaluate the effect of the shunt occlusion on the patient’s hemodynamic status.
As a temporary measure, occlusion of the stent with a balloon for 48 hours has been described; however, this procedure is associated with a high risk of recurrent variceal bleeding and PVT. More acceptable techniques are described below.
One technique for reduction of TIPS flow is that of placement of a self-expanding stent graft placed in parallel with a balloon-expandable stent (Fig. 11.14). To accomplish this, two long sheaths are placed via the internal jugular vein, one to deploy the stent graft and the second to insert the balloon-expandable stent. Once the stent graft is deployed, within the existing TIPS stent, the balloon-expandable stent is deployed between the new just-deployed stent graft and the prior TIPS stent. The balloon-expandable stent causes an hourglass configuration of the new stent graft, thereby limiting flow. If the flow is too restricted by the stent, it can be crushed by inflating a balloon inside the new TIPS graft.
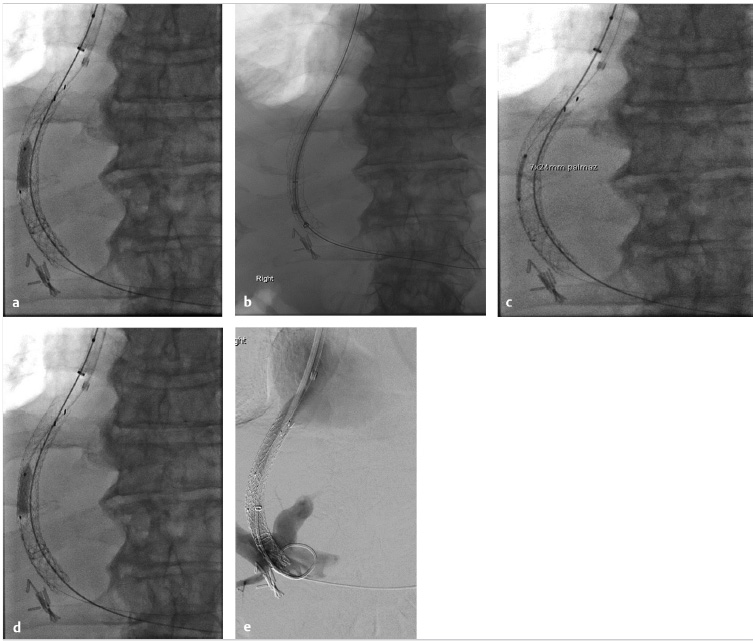
Another technique for TIPS reduction involves placement of a suture-constrained self-expandable stent graft within the shunt. A suture is tied midstent to create an hourglass-shaped stent. The suture should be placed to match with the portion of the TIPS shunt across the liver parenchyma. Other techniques include placement of two parallel stents within the TIPS followed by coil embolization of one of the stents, or deployment of a balloon-expandable bare metal stent or a balloon-expandable stent graft within the TIPS for minor narrowing of the lumen.
To permanently occlude a TIPS, coils and Amplatzer plugs can be safely used once the patient’s hemodynamic stability is confirmed with temporary balloon occlusion.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree