The Anterior Communicating Artery Complex
11.1 Case Description
11.1.1 Clinical Presentation
A 37-year-old woman with long-standing chronic hydrocephalus presented with an incidental unruptured anterior communicating artery (AComA) aneurysm.
11.1.2 Radiologic Studies
See ▶ Fig. 11.1.
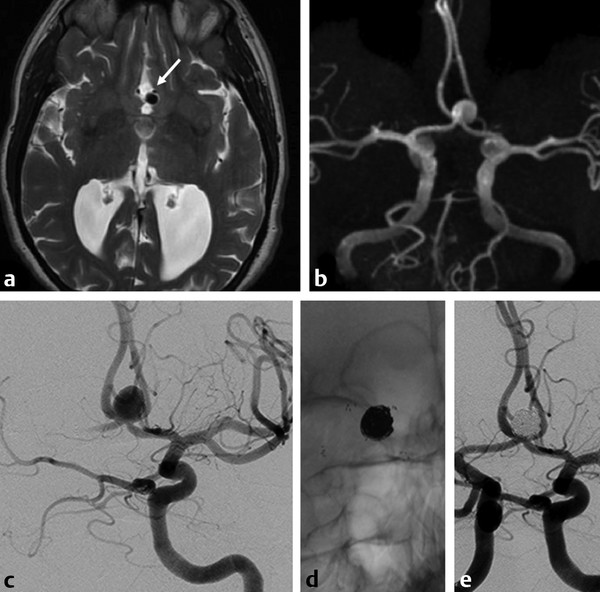
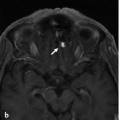
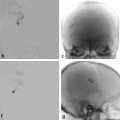
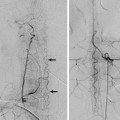
Fig. 11.1 Axial T2-weighted MRI (a) shows dilated ventricles and a prominent flow void in the AComA region (white arrow). Time-of-flight magnetic resonance angiography (b) and left internal carotid artery (ICA) angiogram in working projection (c) reveal a broad-necked AComA aneurysm. Plain radiography (d) and simultaneous bilateral carotid injections (d) after X-stenting and coiling show complete occlusion of the aneurysm. The decision to perform an X-stent-assisted coiling was made to preserve the patency of the AComA.
11.1.3 Diagnosis
AComA aneurysm.
11.2 Anatomy
The most widely used classification of anterior cerebral artery (ACA) anatomy to date is the one proposed in 1938 by Fischer. It defines five segments: A1, the precommunicating segment; A2, the segment that runs below the genu of the corpus callosum; A3, the segment that runs around the genu of the corpus callosum; and A4 and A5, which are considered the terminal portions of the ACA. Given that it has become common language in the neurovascular anatomy, this nomenclature is used in this section.
The right and left A1 segments join to form the AComA in the midline in the lamina terminalis region, above the optic chiasm (70%) or, less frequently, above the cisternal segment of the optic nerves (30%). The “classic” anatomy, in which two equal-sized A1 segments form the AComA, is seen in only 20% of cases. Most commonly, one A1 segment will be dominant and bifurcate into the two A2 segments. In these cases, the AComA will be defined by the entry point of the hypoplastic A1. Complete absence of the A1 segment does not occur.
The AComA complex gives rise to small perforating vessels that are highly variable. Most commonly, these arteries will arise from the medial part of the AComA when the A1 segments are symmetrical, and ipsilateral to the dominant A1 when the segments are unequal. In cases of fenestrated or duplicated AComA, both limbs will have perforators arising from each limb. Three major groups of perforators have been described: the hypothalamic arteries that supply the lamina terminalis, the anterior hypothalamus, and the infundibulum of the pituitary; the chiasmal branches; and the subcallosal branches, which supply the rostrum and genu of the corpus callosum, the anterior cingulate gyrus, the septum pellucidum, and both columns of the fornix. The subcallosal artery is usually the largest, and often it is a single branch, whereas the other groups of perforators often consist of multiple small branches.
The anatomy of the medial lenticulostriate arteries arising from the ACA and of the recurrent artery of Heubner is discussed in Case ▶ 14. Multiple variations of the AcomA complex and its adjacent vessels can be identified.
11.2.1 A1 Segment Fenestration
Fenestrations of the A1 segment are rare, with an estimated prevalence of 0 to 4% in anatomic studies and less than 0.1% in angiographic studies (▶ Fig. 11.2). These fenestrations may be caused by the optic nerve coursing through the fenestration, which can be explained by the same considerations used to explain the infraoptic course of the A1, as outlined in the previous chapter; in most instances, though, a fenestration of the A1 segment is related to incomplete obliteration of anastomoses of a primitive vascular network. An association between A1 fenestrations and proximal A1 aneurysms has been proposed, but it is mostly based on isolated case reports.
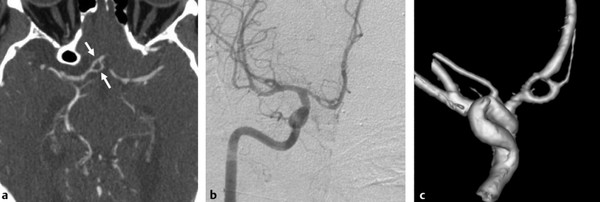
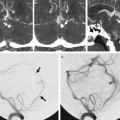
Fig. 11.2 A 60-year-old man presented for postoperative arteriovenous malformation assessment. Axial CTA (a) reveals a fenestrated supraoptic A1 segment (arrows). Right ICA angiogram in anteroposterior view (b) and 3D rotational reconstruction (c) better depict the anatomic variation.
11.2.2 AComA Fenestration
The AComA develops from a multichanneled vascular network during the embryologic stage that coalesces in various degrees by the time of birth. This process is responsible for the wide variety of AcomAs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree