12 Venous Anomalies and Syndromes: Classifications, Evaluation, and Treatment
Summary
Venous malformations and their classification, evaluation, and treatment tend to remain poorly understood by the medical community at large. They represent a small niche in interventional radiology practice, but patients with venous anomalies will often ultimately present to interventional radiologists after evaluation and sometimes treatment by other specialists. An understanding of venous anomalies and their management is beneficial to interventional radiologists, both for purposes of managing these patients when appropriate and for understanding when to refer to a specialist with greater experience in management of these sometimes-complex patients. This chapter will review patient presentations, radiographic and clinical evaluation, and procedural details, introducing concepts related to potential outcomes and complications of treatment.
12.1 Introduction
Venous malformations (VMs) are dilated venous channels or sacs filled with venous blood that may or may not communicate with the systemic venous system. A dysfunctional smooth muscle component leads to gradual expansion of VMs over time. 1 VMs are the most common type of vascular anomaly and can be found in isolation or in the setting of a genetic syndrome. 2 When superficial, VMs are often soft, compressible, bluish lesions. VMs can involve multiple body parts and can cross tissue and anatomic planes extending into adjacent muscles and bones, as well as along vasculature and nerves. The proper management of VMs begins with accurate diagnosis and evaluation for the extent of disease prior to selecting a targeted interventional therapy or surgical approach. 1 , 3 , 4 Pediatricians, interventionalists, dermatologists, and surgeons who diagnose and treat VMs should be well versed in the classification, imaging evaluation, and treatment options available for venous anomalies. In this chapter, we outline the currently accepted classification schemas, radiographic workup, and treatment options for a variety of VMs and venous syndromes.
12.2 Case Vignette
12.2.1 Patient Presentation
An 8-year-old boy presented with a palpable mass of his right forearm reported as intermittently painful. His mother stated that he was born with the lesion but it had grown in size over time. The lesion had first become symptomatic at age 5. He reported pain after applying pressure to the area and also with prolonged activity. The lesion did not change in size when dependent.
12.2.2 Physical Exam
Vital signs were normal. Focused exam of the right upper extremity revealed a 4 × 5 cm area of soft tissue swelling without discoloration over the proximal volar forearm. The lesion was painful to palpation but did not increase in size when placed in a dependent position. There was no palpable pulsation or bruit. Sensory and motor exam of the right lower extremity were unremarkable.
12.2.3 Laboratory Workup
Notable values included:
D-dimer 2.07 mg/L (less than five times elevated from normal limits).
Fibrinogen: 323 mg/dL (normal).
12.2.4 Imaging
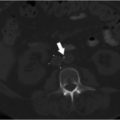
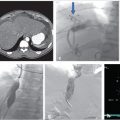
Multiphase and multiplanar MRI with and without contrast was obtained. MRI revealed a lobulated and multiseptated lesion with tubular foci within the medial belly of the brachioradialis muscle. The lesion was T2 hyperintense with T2 hypointensities scattered throughout, likely representing phleboliths. There were also several fluid–fluid levels, representative of “hematocrit layering,” hemorrhage, or proteinaceous material. After administration of intravenous contrast, the lesion demonstrated uniform enhancement similar in timing and appearance to the surrounding venous structures. There was no evidence of osseous invasion. Findings were compatible with solitary VM (Fig. 12.1a,b).

12.2.5 Specifics of Consent
Treatment alternatives, including compression and surgical excision, were discussed, reviewing the limitations and risks of these alternatives. Risks of sclerotherapy discussed with the patient and parents included the following: thrombosis, which may be localized with associated postprocedural pain, may extend to the deep venous system with outflow occlusion or embolize with resultant, possibly significant pulmonary embolism, and, more rarely, disseminated intravascular coagulation (DIC); extravasation of sclerosant with soft tissue damage; entry of sclerosant into the deep venous system with resultant endothelial damage; allergic reaction to sclerosant; swelling of treated area with risk for compartment syndrome in the extremity; development of extremity contracture due to immobilization postprocedure; nerve damage with loss of sensory or motor function; and failure of procedure. Expectations were also set relative to the likelihood of repeat procedures for control of the chronic disorder.
Details of Procedure
Informed consent was obtained and all patient questions answered.
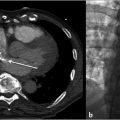
Preprocedural ultrasound revealed multiple hypoechoic tubular structures in the region of the previously identified VM without appreciable flow (Fig. 12.2a,b).
The right forearm was prepped and draped in usual sterile fashion at the location of the VM.
Ultrasound guidance was used to percutaneously access the VM in the right forearm/brachioradialis muscle at two sites with 21-gauge needles (Fig. 12.2c).
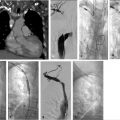
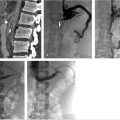
Presclerotherapy venograms were performed at each access site to characterize the VM, assess size, and evaluate for presence and size of draining veins (Fig. 12.3a).
An additional 21-gauge needle was placed into the cephalad aspect of the VM for decompression during sclerotherapy (Fig. 12.3b).
Sotradecol foam mixture was prepared using a ratio of 1-mL 3% Sotradecol, 0.5-mL Lipiodol, 4-mL air.
Sotradecol foam was injected into each site under fluoroscopic guidance using negative contrast/contrast displacement technique (Fig. 12.3c,d).
After allowing 15 minutes for the Sotradecol to act, the needles were tested for further blood return, and finding none they were removed and manual pressure applied until hemostasis was achieved. Postprocedural ultrasound revealed increased echogenicity and acoustic shadowing at the site of treatment (Fig. 12.2d).
The patient was admitted overnight for pain control, management of postprocedure swelling, and neurovascular checks.
The patient was discharged the next morning without complication.

Fig. 12.2 Intraprocedural ultrasound imaging of the same 8-year-old male with a tender mass in his right forearm. (a) Preprocedural ultrasound revealed multiple hypoechoic tubular structures in the right forearm. (b) Color Doppler ultrasound demonstrated no flow appreciable flow, compatible with a venous malformation. (c) Ultrasound-guided percutaneous access into the lesion. (d) Postsclerotherapy ultrasound demonstrates increased echogenicity and acoustic shadowing at the site of sclerosant injection. 
Fig. 12.3 Intraprocedural fluoroscopic images of the same 8-year-old male with a tender mass in his right forearm. (a) Access into the first site of the VM with preprocedural venogram defining the extent of the first site. (b) Access into the second site of VM with preprocedural venogram defining the extent of the second site. (c) Placement of a decompression needle within the second site. (d) Postsclerotherapy image.
Follow-up
The patient underwent a right upper extremity duplex ultrasound in the immediate postprocedural period, which revealed a patent deep venous system without evidence of thrombosis. The patient presented for follow-up 3 days after sclerotherapy and reported slightly increased pain at the site of sclerotherapy, compatible with normal postsclerotherapy discomfort. Pain was well controlled with ibuprofen. The patient was referred for physical therapy to maintain range of motion and to prevent contractures. The patient underwent follow-up MRI approximately 2 months postsclerotherapy, which revealed greater than 50% decrease in the size of the lesion (Fig. 12.1c–e). The patient was also seen in clinic at approximately 2 months postsclerotherapy. At this time, the patient reported that he was now asymptomatic with marked improvement in soft tissue swelling of the right forearm. The patient and his family were instructed to follow-up in 1 year with an MRI or sooner if he became symptomatic again.
12.3 Epidemiology and Scope of the Problem
VMs are the most common type of vascular malformation. The estimated incidence of venous anomalies is 2%. Ninety percent of VMs are solitary, which usually indicates that the VM is sporadic. Multiple VMs account for the remaining 10% of lesions and are often related to heritable conditions or syndromes. 1 , 3
12.3.1 Classification
The management of VMs often warrants a multidisciplinary approach; thus, it is important to use a common language between multiple subspecialties in order to properly define and classify these lesions with a high degree of accuracy and precision. 3 Several classification schemas have been developed, dating back to the 1970s. The most widely recognized classification system was developed and refined by the International Society for the Study of Vascular Anomalies (ISSVA). 2 , 4 , 5 Since the society’s founding in 1976 by Mulliken and Young, the ISSVA has been refining its classification system on a biannual basis. The ISSVA classifies vascular anomalies based on cellular features, flow characteristics, associated clinical features and behavior, and genetics. The most recent iteration of this system was adopted in 2014. 5
At the highest level, vascular anomalies are classified as either vascular tumors or vascular malformations (Table 12.1). A vascular tumor results from aberrant cellular proliferation, whereas a vascular malformation is a regional defect in vascular morphogenesis. Vascular tumors are further subdivided into benign, malignant, and aggressive. Similarly, vascular malformations are subdivided into low-flow and high-flow lesions (e.g., venous, lymphatic vs. arterial and arteriovenous malformation), and also whether it is simple (one vascular malformation in one lesion) or combined (two or more vascular malformations in one lesion) (Table 12.1). 5 With respect to VMs, the ISSVA classification schema for simple and combined VMs is shown in Table 12.2 and Table 12.3, respectively. The ISSVA also provides a table of associated genes (Table 12.4) and additional anomalies associated with VMs (Table 12.5). 5
Simple venous malformations |
Common VM |
Familial VM cutaneomucosal |
Blue rubber bleb nevus (Bean) syndrome VM |
Glomuvenous malformation |
Cerebral cavernous malformation |
Others |
Abbreviation: VM, venous malformation. |
Venous malformation | Gene |
Common VM | TIE2 somatic |
Familial VM cutaneomucosal | TIE2 |
Glomuvenous malformation | Glomulin |
Cerebral cavernous malformation | KRIT1/Malcavernin/PDCD10 |
Abbreviation: VM, venous malformation. | |
Another tool that aids in the classification of vascular anomalies is the S.E. Mitchell Vascular Anomalies Flow Chart (SEMVAFC) (Fig. 12.4). 3 The SEMVAFC uses clinical symptoms, physical exam findings, and imaging to provide a clear pathway for physicians to accurately diagnose a vascular abnormality. In order to preserve a common language among the multidisciplinary team of physicians who manage these lesions, SEMFAFC is broken up into similar categories to the ISSVA classification schema. For instance, VMs are classified as slow-flow malformations under SEMVAFC. 3 Of note, the term cavernous hemangioma has previously been used to refer to VMs; in fact, this term should only be used to refer to deep hemangiomas, and cavernous hemangioma should no longer be associated with VMs.

Puig and colleagues developed a classification system specific to VMs using phlebography to help guide interventional therapy. 6 This classification is based off venous drainage of the VM and was meant to estimate the risk of central embolization, or the risk of the sclerosant agent reaching the central vasculature and causing a complication such as pulmonary vasospasm or direct cardiotoxicity. Type 1 malformations are isolated without any peripheral drainage. Type II malformations drain into “normal” veins. Type III malformations drain into dysplastic veins. Finally, type IV malformations are venous ectasia. The authors found that patients with type I and II VMs had almost no risk of central embolization. However, patients with type III and IV VMs had a much higher risk of central embolization due to their large draining venous channels. 6
12.3.2 Patient Presentation and Evaluation
Sporadic VMs
Superficial VMs can manifest as small varicosities or bluish mass lesions. Deeper VMs often present as soft tissue masses. Both superficial and deep VMs are typically soft and easily compressible. The lesions can sometimes enlarge with Valsalva’s maneuver or when the affected area is in a dependent position due to blood pooling. 1 , 3 , 7 While VMs most commonly present during mid-to-late childhood and grow in size as time progresses, they are often present at birth. VMs typically grow as the patient grows; however, rapid growth occurs during peak hormonal levels (i.e., puberty, pregnancy, and menstrual cycles). VMs most commonly are found on the head and neck (47%), extremities (30%), and trunk (13%), but can occur anywhere on the body. As discussed above, the majority of VMs are solitary; however, when multiple VMs are present, there should be a high index of suspicion for an underlying syndrome. 1 , 2
Most VMs remain asymptomatic over a patient’s lifetime. When symptomatic, however, they often present with pain secondary to focal thrombosis or due to mass effect. Commonly, pain can be worse in the morning due to pooling and stasis of blood within the VM during sleep or worse after physical activity due to pooling from increased venous pressure. 1 , 3 , 8 , 9 VMs can also impair function when they invade or impinge upon muscle groups, joints, and nerves. Both superficial and deep VMs can cause varying degrees of cosmetic deformity. Head and neck VMs can also cause problems with respiration, speech, swallowing, and vision. 7 , 10 , 11 , 12
Venous Syndromes
Klippel–Trénaunay (KT) Syndrome
KT syndrome is characterized by VMs/varicosities, capillary–lymphatic–VMs, and bony/soft tissue hypertrophy of the limbs, which is often unilateral. 13 , 14 , 15 Though KT syndrome is thought to be a genetically sporadic syndrome, genetic associations are still being explored. With regard to the venous component of this disorder, patients present with superficial and deeper varicosities and malformations that may benefit from sclerotherapy. It is important to note that the deep venous system must be evaluated prior to intervention, since patients with KT syndrome can have absent or hypoplastic deep venous systems. 1 , 2 , 13
Blue Rubber Bleb Nevus Syndrome (BRBNS)
BRBNS, also called Bean’s syndrome, is a sporadic syndrome characterized by multiple superficial skin VMs as well as small and large bowel VMs. Due to the presence of multiple gastrointestinal VMs, these patients are at risk for gastrointestinal bleeding. 16
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree