13 Kidneys
Ultrasound (US) is the workhorse in imaging of the urinary tract. The US examination should ideally be performed by an investigator either experienced in pediatric US or supervised by a pediatric radiologist and using an up-to-date protocol for pediatric imaging. The child should be well hydrated, and a pre- and postvoid assessment must be included. The examination is performed with both curved and linear array, high-frequency transducers. Start with an assessment of the bladder shape, wall, and neck, and try to identify the ureter ostium and distal ureters. Proceed with the retrovesical space, where the internal genitalia can be visualized behind the full bladder. Assessment of the kidneys both before and after micturition is important in order to detect changes in the collecting system after voiding. The bladder must also be assessed before and after micturition, and the residual urine volume should be measured. The kidneys are best seen from the back, and scanning the patient in the prone or decubitus position will give the most detailed images of the renal parenchyma when both curved and linear transducers are used. If there is dilatation of the collecting system, measurements of the maximal width of the renal pelvis in the transverse plane, the maximal calyx diameter, and the narrowest parenchymal width should be provided. It is important to obtain measurements of the kidney in three planes. For serial measurements (e.g., in growth assessment), consistency is crucial, and measurements should ideally be obtained by scanning the patient from the back and using the same type of transducer every time (i.e., either curved or linear array transducer). Amplitude-coded Doppler and duplex Doppler are optional techniques to assess the renal vasculature and kidney perfusion, to differentiate prominent hilar vessels from the renal pelvis, and to depict the urine jet from the ureter ostium.
Tips from the Pro
Always start the examination with an assessment of the bladder to ensure proper pre-micturition images. This is particularly important with children who are still in their nappies.
Adjust the gain behind the bladder; otherwise, it is easy to miss dilated ureters.
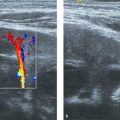
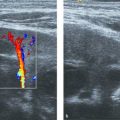
Color Doppler can be applied to differentiate prominent hilar vessels from the renal pelvis (Fig. 13.1 c).
Do not forget to assess the kidneys from behind the back to obtain detailed images of the renal parenchyma.

13.1 Normal Anatomy and Variants
13.1.1 Kidneys
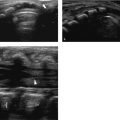
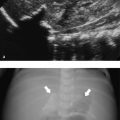
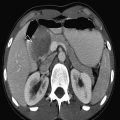
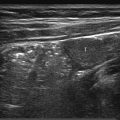
The normal sonographic appearances of the kidneys vary with age (Table 13.1). In the neonate, the glomeruli occupy twice as much of the cortical volume as in adults, and medullary volume is larger in children. Therefore, the renal cortex in the neonate is isoechoic to the liver and spleen, and the relatively high echogenicity of the renal cortex contrasts with the prominent, hypoechoic medullary pyramids (Fig. 13.1 a, b). In premature infants, the renal cortex is even hyperechoic to the liver and spleen. The echogenicity of the renal cortex decreases gradually in the first year of life, and the cortex usually becomes hypoechoic to the liver when the child is 12 to 15 months old (Fig. 13.2). Persistent fetal lobulation is a common finding in infants (Fig. 13.1 a). In infants and young children, the renal sinus lacks the echogenicity seen in adults and older children because of the paucity of fat (Fig. 13.3).


In the neonate, protein deposits may cause transient increased echogenicity at the tip of the medullary pyramids (Fig. 13.4). In children with normal urine output, this is a self-limiting finding that disappears in a few days and does not require further work-up.

A renal pelvis up to 10 mm in anteroposterior diameter is a normal finding in children and should not automatically be labeled as pathologic dilatation.
Fetal lobulation of the kidneys may persist through adulthood and can be differentiated from renal scarring by the lack of parenchymal thinning. Renal scars are most often located over the calices, whereas the lobules in fetal lobulation contain a pyramid, so the lobulation lies between the pyramids.
Cortical fusion defects may be seen as interrenuncular septa, which are hyperechoic lines extending from the cortex to the medulla of the kidney (Fig. 13.5). There is no associated parenchymal thinning.

A column of Bertin is a normal structure and represents the extension of renal cortical tissue that separates the pyramids. Occasionally, a prominent or hypertrophic column of Bertin may be seen in the middle part of the kidney and should not be mistaken for a renal tumor. The hypertrophic Bertin column is continuous with and similar in appearance to the renal cortex. The column may splay the calices, but the outline of the kidney is preserved (Fig. 13.6).

Compound calices can be seen in the upper and lower poles of the kidney as small, elongated, fluid-filled calices (Fig. 13.7). They occur when more than one papilla drains into a single calyx. Compound calices have no mass effect, there is no dilatation of the calyx neck, and there is no thinning of the overlying renal cortex.

Duplex systems are a common finding, with a reported incidence of 0.8%. Duplications range from a split renal pelvis to a complete duplication, with two separate ureters and two bladder ostia. The ureters often fuse somewhere distal to the renal pelvis to form one ureter (Fig. 13.8). Duplicated systems are more often associated with urinary obstruction or vesicoureteral reflux. In the absence of complications, such as recurrent or atypical urinary tract infections or dilatation, a duplex system may be regarded as a normal variant and does not require further work-up or treatment.

The normal waveform of the renal artery on pulsed Doppler sonography is shown in Fig. 13.9. Both peak systolic velocity and the resistive index (RI) of a normal kidney are age-dependent. Peak velocity in the main renal artery varies from 30 to 50 cm per second in the neonate to 100 to 110 cm per second in the adolescent. An RI up to 0.85 is normal in a neonate and decreases to less than 0.7 during the first year of life. The renal arteries course posterior to the renal veins and reach the renal hilum, where they branch to form segmental arteries. It is not uncommon to have accessory polar arteries to the kidney; therefore, Doppler curves must be obtained in all segments of the kidney.

13.1.2 Ureters
Nondilated ureters can be difficult to assess throughout their entire length; however, even normal ureters can often be visualized behind the bladder in children (Fig. 13.10). The ureters should be thin-walled and have a maximum diameter of 5 mm. Normal peristalsis of the ureters and the presence of a urine jet from the ureter ostia within the bladder are useful parameters for the evaluation of ureteral function and patency.

13.1.3 Bladder
The bladder can be properly assessed only when the bladder is full; therefore, proper hydration and filling of the bladder before the scan are important. The bladder wall is smooth, and the musculature at the trigone is slightly thicker than in the rest of the bladder. The bladder wall thickness should not exceed 3 mm when the bladder is full and 5 mm when it is empty. Bladder emptying and residual urine should be correlated with the patient’s sex, age, and bladder volume before micturition.
Subjects | Longitudinal midclavicular dimension of the right kidney (mm) | ||||||||
Age range (months) | Number | Mean | SD | Minimum | Maximum | Percentile | Suggested limits of normal | ||
5th | 95th | Lowermost | Uppermost | ||||||
1–3 | 50 | 50 | 5.8 | 38 | 66 | 40 | 58 | 35 | 65 |
4–6 | 39 | 53 | 5.3 | 41 | 66 | 50 | 64 | 40 | 70 |
7–9 | 17 | 59 | 5.2 | 50 | 70 | 52 | 66 | 45 | 70 |
12–30 | 18 | 61 | 3.4 | 55 | 66 | 55 | 65 | 50 | 75 |
36–59 | 22 | 67 | 5.1 | 57 | 77 | 59 | 75 | 55 | 80 |
60–83 | 26 | 74 | 5.5 | 62 | 83 | 65 | 83 | 60 | 85 |
84–107 | 32 | 80 | 6.6 | 68 | 93 | 70 | 91 | 65 | 95 |
108–131 | 27 | 80 | 7.0 | 69 | 96 | 69 | 89 | 65 | 100 |
132–155 | 89 | 6.2 | 6.2 | 81 | 102 | 82 | 100 | 70 | 105 |
159–179 | 22 | 94 | 5.9 | 83 | 105 | 85 | 102 | 75 | 110 |
180–200 | 11 | 92 | 7.0 | 80 | 107 | 83 | 102 | 75 | 110 |
Subjects | Longitudinal midclavicular dimension of the left kidney (mm) | ||||||||
Age range (months) | Number | Mean | SD | Minimum | Maximum | Percentile | Suggested limits of normal | ||
5th | 95th | Lowermost | Uppermost | ||||||
1–3 | 50 | 50 | 5.5 | 39 | 61 | 42 | 59 | 35 | 65 |
4–6 | 39 | 56 | 5.5 | 44 | 68 | 47 | 64 | 40 | 70 |
7–9 | 17 | 61 | 4.6 | 54 | 68 | 54 | 68 | 45 | 75 |
12–30 | 18 | 66 | 5.3 | 54 | 75 | 57 | 72 | 50 | 80 |
36–59 | 22 | 71 | 4.5 | 61 | 77 | 61 | 76 | 55 | 85 |
60–83 | 26 | 79 | 5.9 | 66 | 90 | 70 | 87 | 60 | 95 |
84–107 | 32 | 84 | 6.6 | 71 | 95 | 73 | 93 | 65 | 100 |
108–131 | 27 | 84 | 7.4 | 71 | 99 | 75 | 97 | 65 | 105 |
132–155 | 15 | 91 | 8.4 | 71 | 104 | 77 | 102 | 70 | 110 |
159–179 | 55 | 96 | 8.9 | 83 | 113 | 84 | 110 | 75 | 115 |
180–200 | 11 | 99 | 7.5 | 87 | 116 | 90 | 110 | 80 | 120 |
Abbreviation: SD, standard deviation. Note: This study included 307 pediatric subjects (169 girls and 138 boys) ranging in age from full-term newborns (5 days) to 16 years. The subjects were imaged in the supine or slightly right/left lateral decubitus position. The renal measurements were obtained in the coronal plane at the level of the hilum. | |||||||||
13.2 Congenital Anomalies of the Kidney and the Urinary Tract
Congenital anomalies of the kidney and urinary tract cover a broad range of disorders (see box Congenital anomalies of the kidney and urinary tract ) that result from the following abnormal renal developmental processes:
Malformation of the renal parenchyma resulting in failure of normal nephron development, as seen in renal agenesis, renal dysplasia, and multicystic dysplasia;
Abnormalities of embryonic migration of the kidneys, as seen in renal ectopia (e.g., pelvic kidney) and fusion anomalies, such as horseshoe kidney;
Abnormalities of the developing urinary collecting system, as seen in duplicate collecting systems, posterior urethral valves, and ureteropelvic junction obstruction.
Although in many cases congenital anomalies of the kidney and urinary tract occur in the context of multiple-organ malformation syndromes, most cases are nonsyndromic. Defects can be bilateral or unilateral. The inheritance pattern may be variable, and different congenital anomalies of the kidney and urinary tract may be present in the same patient or in different members of the same pedigree.
The main ultrasound feature is dilatation of the urinary tract (see box Role of ultrasound in the assessment of a dilated urinary tract ). Prenatal imaging is useful to select patients for postnatal work-up. Bladder and urethral pathologies and multicystic kidneys are described in later sections of this chapter.
Congenital anomalies of the kidney and urinary tract
Aplastic–hypoplastic kidney
Multicystic kidney
Ureteropelvic junction obstruction
Ureterovesical junction obstruction
Ureterovesical reflux
Duplex systems
Ectopic ureter
Bladder and urethra anomalies
Horseshoe kidney
Role of ultrasound in the assessment of a dilated urinary tract
Detection of dilated urinary tract segments
Grading of hydronephrosis
Evaluation of the nature of an obstacle (stenosis, stone, fungus ball, tumor)
Evaluation of the renal parenchyma (thickness, structure)
Identification of complications (hydropyonephrosis, urine extravasation, others)
13.2.1 Renal Hypodysplasia
Although the term dysplasia is based on a histologic evaluation, it is often used to define cases with abnormal renal parenchymal structure—unilateral or bilateral, diffuse or focal—associated with a reduction of the kidney size. It is characterized by inhomogeneous hyperechoic parenchyma with less evident or absent corticomedullary differentiation. A common finding is the presence of isolated cysts, usually small (diameter < 1 cm) in the outer, often subcapsular, cortex (Fig. 13.11 and Fig. 13.12).


13.2.2 Ureteropelvic Junction Stenosis
Ureteropelvic junction stenosis is the most common type of obstruction of the upper urinary tract in childhood. It is often bilateral and may differ in degree between the two kidneys; it is frequently demonstrated in prenatal US scans. The stenosis may be intrinsic or due to external compression, often as the result of a supernumerary renal artery.
US features are often specific, with dilatation of the pelvicaliceal system in which the renal pelvis is more enlarged than the calices and in which a clear “stop” is seen at the ureteropelvic junction (Fig. 13.13) without dilatation of the ureter.

In case of a suspected crossing vessel on color Doppler US (Fig. 13.14), a more detailed anatomical study by means of magnetic resonance (MR) angiography is often necessary to plan the surgical approach in terms of risk and scheduled time in the operating theater.

13.2.3 Ureterovesical Junction Stenosis
Ureterovesical junction stenosis is a defect of the ureterovesical junction, usually unilateral. It is also associated with vesicoureteral reflux (VUR) and urinary tract infections (UTI). The whole collecting system on the affected side is dilated, with the lower part more markedly affected than the upper. It is sometimes possible to visualize the premural stenotic ureteral tract (Fig. 13.15). The ureter is often abnormally dilated and tortuous, and uroepithelial thickening can be seen (Fig. 13.16).


13.2.4 Ureterovesical Reflux
Ureterovesical reflux is a consequence of dysfunction in the ureterovesical junction. It is the most common urological anomaly associated with recurrent UTI in childhood and may cause renal scarring leading to reflux nephropathy (Fig. 13.17). US can show various degrees of hydroureteronephrosis, which tends to increase during crying, micturition, or other maneuvers that increase abdominal pressure. Thickened uroepithelium is often seen in reflux systems.

It may be possible to diagnose VUR on color Doppler US when backward flow of urine toward the ureter and the pelvis is detected (Fig. 13.18). However, normal US findings do not exclude the presence of VUR, even if it is moderate or severe. Patients with UTI must be selected for further work-up for VUR with voiding cystourethrography (VCUG) based on the clinical presentation of the infection, the patient’s age and family history, and the US findings (see section on UTI). Severe and early antenatal VUR in male fetuses is often associated with high-degree renal dysplasia, which can lead to progressive loss of renal function.

13.2.5 Duplicate Collecting System
This is a quite common, often bilateral, urologic anomaly. Duplicate collecting systems (also known as duplex collecting systems) can be defined as renal units containing two pyelocaliceal systems. The US feature is the presence of a midrenal parenchymal septum dividing the two moieties. Indirect US findings are increased renal size (elongated “bread stick” appearance) and contour notches (Fig. 13.19).

The collecting systems may fuse anywhere along their course to form a single ureter, or they may empty separately into the bladder (Fig. 13.20). Dilatation of one or both ureters is a frequent finding. VUR is common, involving the lower collecting system, while ureterocele is commonly associated with the upper pole ureter (Fig. 13.21). Ureterocele is a cystic dilatation of the intravesical submucosal ureter that changes in size with ureteral peristalsis/ micturition.


US shows an echogenic membrane arising from the bladder wall. This membrane is more or less rounded, owing to differences in internal tension (Fig. 13.22). Ureteroceles may have an ectopic orifice in the bladder neck or urethra (Fig. 13.23 and Fig. 13.24). Ectopic ureters and ureteroceles may also be encountered within a single excretory system.



13.2.6 Horseshoe Kidney
This anomaly is included in a group of so-called fusion anomalies, in which the two kidneys are fused in early embryonic life. Fusion anomalies of the kidneys can generally be grouped into two categories: (1) horseshoe kidney and its variants; and (2) cross-fused ectopy.
Horseshoe kidney is the most common fusion anomaly. It consists of two distinct functioning kidneys on each side of the midline, connected at the lower poles by an isthmus of functioning renal parenchyma or by fibrous tissue that crosses the midline of the body. Horseshoe kidneys are usually ptotic, meaning that the distance between the posteroinferior pilaster of the diaphragm and the upper renal pole is 3 cm or more. The isthmus is located in front of the inferior cava vein, aorta, and rachis (Fig. 13.25 a). VUR and/or ureteropelvic junction stenosis is more frequently seen in this anomaly (Fig. 13.25 b).

Horseshoe kidney is generally distinguished from cross-fused ectopia, in which the two fused kidneys lie on one side of the spine and the ureter of the crossed kidney crosses the midline to enter the bladder on the opposite side. Therefore, the presence of a ureter behind the bladder and the absence of an ipsilateral kidney combined with an elongated contralateral kidney is highly suggestive of cross-fused ectopia.
13.3 Urolithiasis and Nephrocalcinosis
Urolithiasis is the presence of stones in the urinary collecting system. It is the result of a complex interaction between the environment (stone-promoting and stone-inhibiting factors) and heredity (Table 13.2). The prevalence of urinary stones varies by region, being more frequent in the Far and Middle East and less common in North America and Europe (1–5 cases per 10,000 children). Unlike infectious stones, which are found mainly in infants and young children, calcium stones increase in incidence from the age of 5 years. Uric acid stones are very rare in childhood.
Promotes stone | Inhibits stone | |
Urinary volume | Low | High |
pH/citrate | Lowa | Higha |
Bacteria | Infected urine | Sterile urine |
Stone-forming elements | Concentratedb | Dilute |
aStruvite stones are an exception because they require an alkaline pH in order to form. bIn the case of hypercalciuria, hyperuricuria, hyperoxaluria, and cystinuria. | ||
The classic adult presentation of sudden acute flank pain is uncommon in pediatric urolithiasis. Hematuria (gross or microscopic) is found in 33 to 90% of children and occurs equally across age groups. UTI is frequently the presenting sign of urolithiasis in preschool-age children.
Diagnostic steps include the following:
Obtaining a thorough patient and family history;
Performing urinary, blood, and whenever possible stone analysis to identify metabolic abnormalities;
Imaging the urinary tract.
In suspected cases of urolithiasis in children, US is always the first and most often the only imaging study required to exclude or confirm renal stones. Sonographically, a calculus within the collecting system appears as a highly reflective structure that may (usually if > 5 mm in diameter) or may not show acoustic shadowing (Fig. 13.26). A twinkle artifact, which is an artifact occurring behind a highly reflective structure such as a urinary tract stone, may aid stone detection, especially if the stone lacks a strong echo or discrete shadowing. The artifact appears as a rapidly fluctuating mixture of Doppler signals (red and blue pixels) that imitate turbulent flow. However, the Doppler spectrum is absolutely flat, a finding that is characteristic of noise (Fig. 13.27).


US of the urinary tract must rule out stasis or an obstruction caused either by a stone or by congenital or acquired abnormalities of the urinary tract (Fig. 13.28 and Fig. 13.29). The typical stone location is within the renal pelvis and/or the renal calices or the ureter, and less often within the bladder. Struvite stones (magnesium ammonium phosphate in a complex with calcium phosphate) and sometimes cystine stones frequently tend to branch and enlarge, often filling the renal calices and assuming a “stag horn” appearance (Fig. 13.30).



The most common ureteral calcifications are stones that migrate down from the kidney (Fig. 13.31) or stones that form locally, primarily in a distal ureteral dilatation (stenotic megaureter; Fig. 13.32). These stones typically become impacted at anatomical sites of narrowing and are especially difficult to detect when they overlie bony structures such as the sacrum. Detection of a ureteral stone via US is difficult; however, the stone may cause obstruction (hydroureter or hydronephrosis) and may be suspected even if not directly visualized.


Non contrast-enhanced, low-dose computed tomography (CT) is more effective than intravenous urography in precisely identifying ureteral stones, and virtually all urinary calculi are visible on CT. However, because of the need for sedation in younger children and the radiation dose, spiral CT is rarely used for detecting urinary tract stones. Low-dose, non contrast-enhanced CT may be indicated, particularly in older children, when a renal stone is strongly suspected and US is negative.
Tips from the Pro
Air in the collecting system may be misinterpreted as urolithiasis. However, shadowing is poorly defined in the case of air; by contrast, it is well defined in urolithiasis.
Vesical intramural bulging at the ureterovesical junction secondary to the endoscopic treatment of ureterovesical reflux may sometimes be confused with a stone! Look carefully at the patient’s clinical history (Fig. 13.33)!

Nephrocalcinosis is microscopic calcification in the tubules, tubular epithelium, or interstitial tissue of the kidney. It typically involves the medullary portion of the kidney (Fig. 13.34). The echogenic pattern may vary according to the underlying tubulopathy, particularly in the initial phase (see box Classification of tubulopathies according to the section of the tubular system involved (p. 17)). This is because different tubular segments may be selectively involved, resulting in different US patterns (Fig. 13.35).
Classification of tubulopathies according to the section of the tubular system involved
Proximal tubules
Fanconi syndrome
Aminoaciduria
Dent syndrome
Proximal tubular acidosis
Idiopathic hypercalciuria
Loop of Henle
Idiopathic hypercalciuria
Bartter syndrome
Distal and collecting tubules
Gitelman syndrome
Liddle syndrome
Distal tubular acidosis


For the detection and monitoring of nephrocalcinosis, high-resolution US is the optimal imaging method.
In a variety of metabolic diseases, urolithiasis and nephrocalcinosis occur together (Fig. 13.36). Medullary nephrocalcinosis is distinguished from both cortical (e.g., in acute cortical necrosis, chronic glomerulonephritis, and chronic graft rejection) and diffuse nephrocalcinosis.

Unlike that of urolithiasis, the clinical presentation of nephrocalcinosis is often asymptomatic, especially during infancy.
Thanks to the routine use of US in premature infants and in children at risk, a large number of conditions are now recognized to be associated with nephrocalcinosis.
Tips from the Pro
Uromodulin deposits within the renal calices in newborns may resemble nephrocalcinosis (Fig. 13.4; Fig. 13.37). These deposits, however, disappear within 1 to 2 weeks, and follow-up will show completely normal kidneys.

13.4 Kidney Cysts and Cystic Nephropathies
Many renal disorders in childhood are characterized by the presence of cysts. A cyst is a dilatation that can involve different segments of the intrarenal collecting system, from glomeruli (Bowman capsules) to distal collecting tubules. The continuity with the nephron may or may not be lost. A cyst is lined by a single layer of flattened epithelium and contains clear serous fluid. There is no communication between the cyst and the collecting system (Fig. 13.38).

Cystic diseases of the kidney include a heterogeneous group of disorders that may be inherited or sporadic, unilateral or bilateral, and symptomatic at birth or detected later in life. Genetically determined cystic disorders are listed in box Classification of genetically determined renal cystic diseases . Renal cysts are also present in many pathologies (Fig. 13.39) and syndromes characterized by dysmorphology or dysfunction of several organs, usually associated with various degrees of renal hypodysplasia. Acquired cysts usually appear in advanced stages of chronic renal failure.
Classification of genetically determined renal cystic diseases
Uromodulin-associated nephropathies (uromodulin renal luminal tubular transfer defects)
Medullary cystic kidney disease
Familial juvenile hereditary nephritis
Ciliopathies (alterations of the cilia or centrosome proteins)
Autosomal-dominant/autosomal-recessive polycystic kidney disease
Nephronophthisis
glomerulocystic kidney disease
Congenital Anomalies of the Kidney and Urinary Tract (CAKUT) alterations in the prenatal developmental process of the kidney and the urinary tracts
Multicystic kidney disease
Renal cystic hypodysplasia

US is the main modality used for imaging cystic kidney disease. MR and CT are complementary techniques in selected cases. Advanced software and transducers with excellent tissue penetration and spatial resolution (multiple-frequency electronic and broadband convex and linear sensors) enable the detection of very small cysts, up to 2 mm in diameter.
The cysts are usually smooth with thin walls unless there are complications (e.g., bleeding, inflammation, abscesses, tumor proliferation). A characteristic sonographic sign is posterior enhancement, which is due to lack of attenuation of the US beam when it crosses the cyst. Lateral acoustic shadows due to interactions of the US beam with the cyst walls are also seen. In some cases, it is important, but sometimes not easy, to exclude a communication with the pyelocaliceal system.
The detailed information gained from US, when combined with clinical data, often leads to a definitive diagnosis. Table 13.3 lists the diagnostic possibilities based on the location of cysts and kidney size.
Cortical cysts | Medullary cysts | |
Renal size decreased | Cystic hypodysplasia | Nephronophthisisa |
Renal size unchanged | Glomerulocystic kidney disease | Nephronophthisisb/medullary sponge kidney disease |
Renal size increased | Autosomal-dominant polycystic kidney diseasec | Autosomal-recessive polycystic kidney diseased |
aAdvanced renal failure. bEarly renal failure. cUbiquitous cysts, mainly cortical. dUbiquitous cysts, mainly medullary. | ||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree