The Middle Cerebral Artery Trunk
14.1 Case Description
14.1.1 Clinical Presentation
A 65-year-old patient with a long-standing history of high blood pressure presented with a stroke in the watershed territory between the lenticulostriate perforators and the penetrating leptomeningeal arteries. Outside magnetic resonance angiography described a filiforme middle cerebral artery (MCA) stenosis, and the patient was scheduled for intracranial percutaneous transluminal angioplasty and stenting
14.1.2 Radiologic Studies
See ▶ Fig. 14.1, ▶ Fig. 14.2.
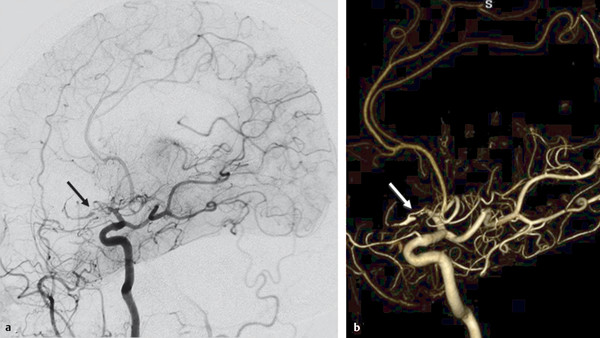
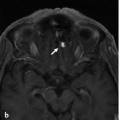
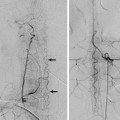
Fig. 14.1 Right ICA angiogram in lateral view (a) and 3D reconstruction (b) shows a short-segmented occlusion of the right proximal MCA. The distal MCA is filled through a small vessel that arises from the proximal MCA and that enters the M1 distal to the segmental occlusion (arrows). There is very slow and incomplete filling of the distal MCA branches. The small-caliber upper limb of this proximal M1 fenestration acts as a natural (but insufficient) bypass. As percutaneous transluminal angioplasty of this small-caliber vessel would have resulted in rupture of the fenestration, the patient underwent extracranial to intracranial bypass surgery to revascularize the distal MCA territory. Case is continued in ▶ Fig. 14.2.
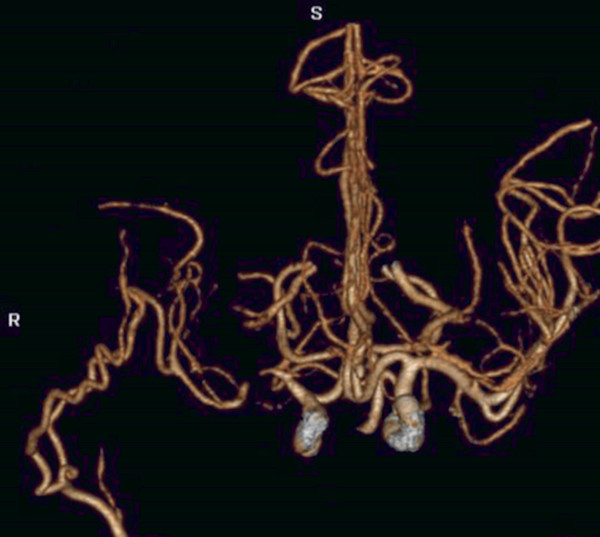
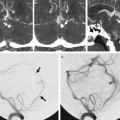
Fig. 14.2 3D CTA demonstrates patency of the extracranial to intracranial bypass, reconstituting the distal right MCA branches.
14.1.3 Diagnosis
Occlusion of the M1 main trunk in the setting of a fenestrated MCA.
14.2 Embryology
The anterior cerebral artery (ACA) is the first and phylogenetically oldest telencephalic vessel and can be considered the terminal branch of the internal carotid artery (ICA). During the first weeks of fetal life, when the embryo is 7 to 12 mm long, lateral striate arteries arise from its wall and provide blood supply for embryonic structures of the telencephalic vesicle that will eventually become the MCA territory. Around the ninth week, after the maturation of the vertebrobasilar system, the MCA develops from the fusion of several ACA perforators of the lateral striate group. This developmental pattern matches that of the recurrent artery of Heubner (RAH). Because both the MCA and the RAH therefore share the same phylogenetic origin, their territories and anatomical variations are related to each other.
Both vessels give rise to perforating centripetal corticostriatal branches supplying, among other territories, the medial putamen, the caudate, and part of the anterior limb of the internal capsule, as is further discussed in Case ▶ 15.
Anatomical variations of the MCA have to be recognized when planning interventions to avoid damage or occlusion of perforating vessels that arise from the MCA and to assess their contribution to the perfusion of the deep MCA territory.
These variations are encountered less frequently than variants of other intracranial arteries and include the fenestrated MCA and the presence of a duplicated or accessory MCA (AMCA). Both types of variations are related to a persistence of transient embryological vessels with a variant pattern of pruning the definitive vessel tree.
14.2.1 Fenestration
A fenestrated MCA arises as a single vessel from the ICA and feeds the MCA territory, but it diverges and reconverges along its course. It is a rare finding, with an incidence of less than 0.5% in angiographic series but as high as 1% in some anatomical series. This variant is usually located in the M1 segment and is often unilateral. A fenestration should not be confused with an early branching of the MCA, with a dissection with a patent pseudolumen, or with a superimposed vessel. The mechanism leading to MCA fenestration remains unclear. In their series of five MCA fenestrations, Gailloud et al. reported an early temporopolar branch arising from the inferior limb of the fenestrated MCA in all cases, suggesting this vessel could play a role in the absent fusion of the early perforating arteries. Perforating branches arise from the upper limb. Although this is a clinically silent anomaly, it is an area of hemodynamic stress and, thus, a site for aneurysmal development, similar to an unfused basilar artery (▶ Fig. 14.3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree