15 Trauma and Fractures: Nonacute Trauma
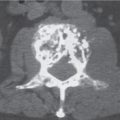
Osteochondritis dissecans appears to be caused in a great majority, if not all cases, by stress, usually of a chronic nature. The knee is the most common site of involvement, with preferential involvement of the medial femoral condyle, followed by the lateral femoral condyle and patella. The disease has also been observed in the distal tibia (tibia plafond), the talar dome, the capitulum of the humerus, and the heads of the femur, humerus, and meta-tarsals, especially the first (Fig. 15.100). The bony fragment may still be located in the corresponding defect of the articular surface or may have become completely separated from the latter and form a loose intra-articular body. The differential diagnosis of loose intra-articular bodies is shown in Table 15.7.
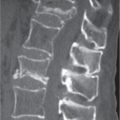
Stress fractures can be subdivided into fatigue fractures and insufficiency fractures. Fatigue fractures occur in normal bones with the application of an abnormal stress or torque caused by a new strenuous or repeated activity. Insufficiency fractures occur when normal stress is placed on an abnormal (osteopenic) bone. Stress fractures are usually symptomatic. They begin as small cortical cracks and may progress to subcortical infraction and eventually a fracture running transversely across the bone. If the fracture line cannot be demonstrated by CT, MRI or a radionuclide examination is always positive and may provide an early diagnosis. MRI has comparable sensitivity and superior specificity to bone scintigraphy in the assessment of stress fractures. Osteomyelitis and bone tumors such as osteoid osteomas can be differentiated from a stress fracture on the basis of both characteristic location and the typical history of stress fractures (Table 15.8 , Fig. 15.101). At a later stage, periosteal reactions with subsequent localized callus formation and cortical thickening obscuring the fracture line are found in the diaphyses of tubular bones. A healing stress fracture limited to the cortex may present as localized cortical thickening that may at times be difficult to differentiate from an osteoid osteoma or healing cortical abscess. However, in the latter two conditions, a central radiolucency may be evident representing the nidus or the abscess, respectively, whereas in the stress fracture, a transverse fracture line may be visible. In an epiphyseal and metaphyseal location (e.g., tibia plateau) and cancellous bone (e.g., calcaneus), a bandlike focal sclerosis usually without appreciable periosteal reaction is more characteristic.
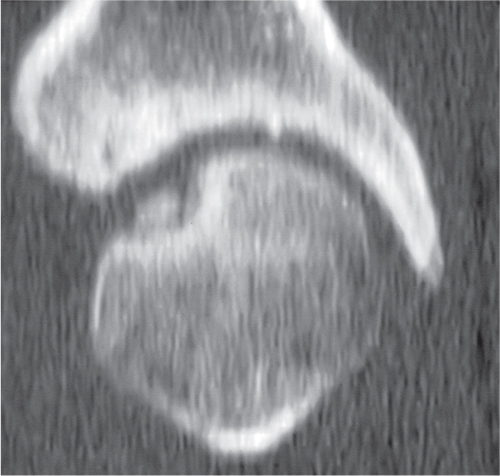
Osteoporosis and rheumatoid arthritis are the two most common conditions in which insufficiency fractures are encountered. Reinforcement lines (bone bars) presenting as well-defined, usually thin sclerotic lines extending partially or completely across the marrow cavity in patients with osteopenia have to be differentiated from poorly defined, bandlike insufficiency fractures. Reinforcement lines can be considered as unmasking of growth arrest lines occurring in childhood, although their pathogenesis is unclear. They are incidental findings without clinical relevance.
Pathologic fractures occur at sites of preexisting abnormalities and are often caused by a minor trauma that would not fracture healthy bone (Fig. 15.102). The differential diagnosis between pathologic and nonpathologic fracture can at times be difficult, particularly when the injury occurred several days to weeks prior to the first imaging examination. In these cases, bone resorption occurring at the site of a nonpathologic fracture may simulate an underlying pathologic lesion and the posttraumatic hematoma a soft tissue extension of the bone lesion. Furthermore, a smaller underlying lytic or sclerotic bone lesion can also be missed by CT, particularly in the presence of displacement at the fracture site. In these cases, the demonstration of other lytic or sclerotic lesions, as well as the absence of a history of trauma or the lack of fracture pain, should suggest the possibility of a pathologic fracture. Pathologic fractures can also occur as early as 5 months after radiation therapy, that is, at a time when the bone does not reveal any radiation-induced changes.
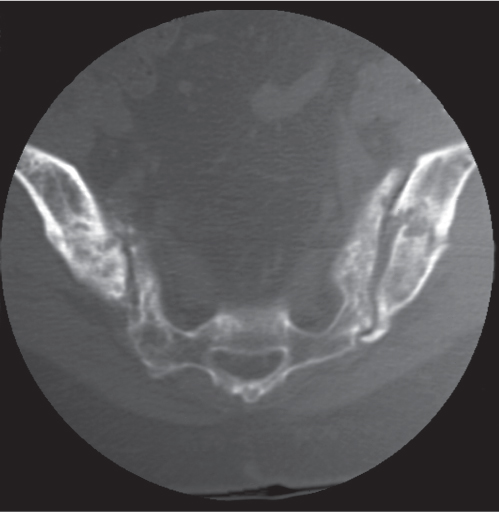
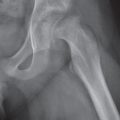
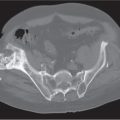
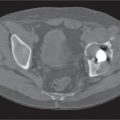
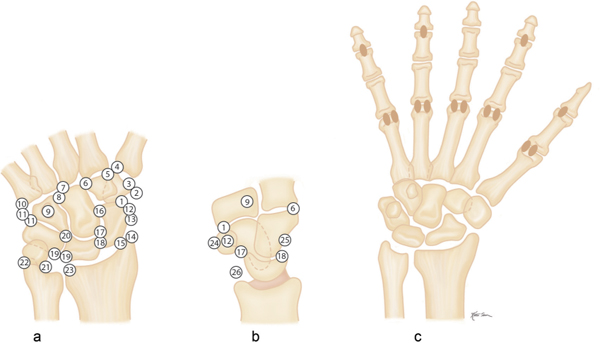
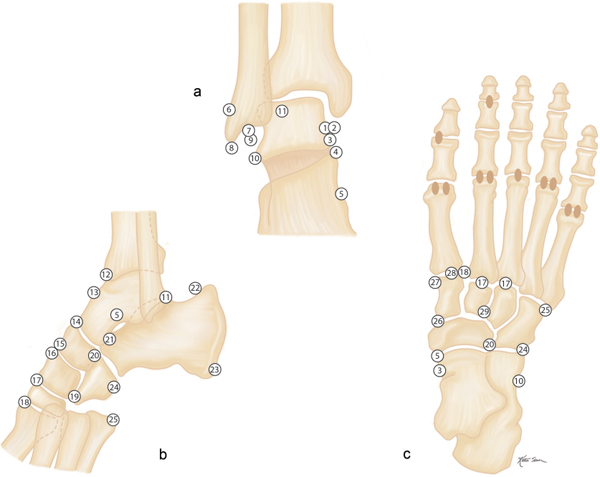
Avulsion fractures at the site of ligament and tendon attachments can be differentiated from accessory ossicles by the lack of a clearly defined cortical margin around the entire bone typical of the latter condition. Furthermore accessory bones have characteristic anatomical locations (Figs. 15.103 , 15.104). The diagnosis of an avulsion fracture can be further supported by the demonstration of a cortical defect or irregularity in the adjacent bone. Avulsion injuries are caused by either a single violent traumatic event or repetitive injuries. They are particularly common in children, as the physeal cartilage of an apophysis is considerably weaker than tendinous or ligamentous tissue. Several avulsion injuries about the pelvis and hips occur in young athletes, including the anterosuperior or anteroinferior iliac spine, iliac crest, ischial tuberosity, pubic symphysis (adductor muscle insertion sites), and greater or lesser trochanter.
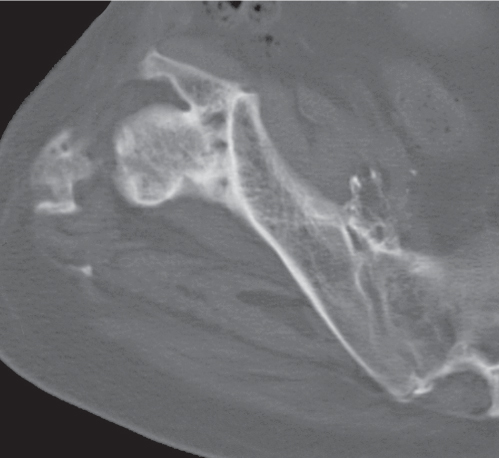
Calcifications and ossifications developing in a ligament after acute or repeated low-grade trauma can be impossible to differentiate from an ossicle, as both may be well corticated. A characteristic location (e.g., Pellegrini–Stieda ossification in an old medial collateral ligament injury of the knee) or the patient’s history (e.g., “rider’s bone” in the adductor muscles of the thigh) may suggest the correct diagnosis.
Heterotopic bone formation in soft tissues following trauma is often termed posttraumatic myositis ossificans (Fig. 15.105), because it occurs most commonly in muscle but is also found in tendons, ligaments, periosteum, and other connective tissues. It frequently develops after insertion of a joint prosthesis, particularly in the hip, and in paraplegic patients. Occasionally, myositis ossificans can mimic a parosteal sarcoma. It can be distinguished from the latter by the fact that its entire periphery is smoothly marginated by a thin cortex (“eggshell” calcification) and by the demonstration of a fine radiolucent line separating the lesion in its entire length from the adjacent bone. A central amorphous calcification associated with a soft tissue mass is not consistent with myositis ossificans and suggests a synovial sarcoma or extraosseous osteogenic sarcoma, where it is present in 30% and 50% of cases, respectively.
Excessive callus formation following a fracture may occasionally have a tumorlike appearance. It is found in fractures that have not been properly immobilized. These fractures may not have been diagnosed because of a decreased sensitivity to pain secondary to a neurologic disorder. Because fractures are not immobilized in abused (battered) child syndrome, excessive callus formation is common in that condition too. Excessive callus formation is also associated in both traumatic and insufficiency fractures of patients with elevated steroid blood concentrations (e.g., Cushing syndrome and steroid therapy). Finally, fractures occurring in osteogenesis imperfecta may heal with excessive callus formation.
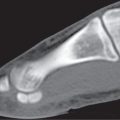
Fractures extending to the articular surface result in a joint effusion (hemarthrosis). A hematocrit effect may be evident in a hemarthrosis characterized by a fluid level caused by the separation of the serum on top of the cellular components of the blood. The demonstration of a fat–fluid level (lipohemarthrosis) in any joint is presumptive evidence of an intra-articular fracture (Fig. 15.106). Pseudofractures (Looser zones, Milkman syndrome) are assumed to be incomplete stress (insufficiency) fractures, presenting radiographically as narrow (2–3 mm) radiolucent bands lying perpendicular to the cortex. At a later stage, sclerosis develops around these lesions, making them more readily detectable. Pseudofractures are present in vitamin D deficiency (osteomalacia and rickets), vitamin D–resistant rickets, hypophosphatasia, renal osteodystrophy, Paget disease, fibrous dysplasia, and hereditary hyperphosphatasia (juvenile Paget disease) or are rarely idiopathic. They are located in the femur (neck and shaft), pubic and ischial rami, scapula, clavicle, ribs, ulna (proximal shaft), radius (distal shaft), metacarpals, metatarsals, and phalanges.
Nutrient arteries pierce the diaphyses of tubular bones obliquely. Their site of entry and angulation are fairly constant, and, characteristically, the vessels point away from the dominant growing end of the bone (the end with the epiphyseal center in short tubular bones or the end with the later fusing epiphysis in long bones). In the long tubular bones of the upper extremity, they run toward the elbow, whereas in the lower extremity, they run away from the knee (“to the elbow they go, from the knee they flee”). Nutrient arteries may be evident as oblique radiolucent cortical channels that should not be confused with fracture lines.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree