16 Male Genital Tract
Diseases of the genital tract are frequent in boys. Common indications for ultrasonography (US) include acute painful swelling of the scrotum or its contents, painless swelling of the scrotum or groin, and absence of detection of the normal testis on physical examination. In this chapter, diseases of the genital tract in boys and the role of US in the diagnosis of these diseases based on US findings will be discussed.
16.1 Technique of Scrotal Ultrasound and Normal Ultrasound Anatomy
The scrotum should be positioned comfortably for a successful US examination. In older children and adolescents, a simple method is to lift the scrotum and close the thighs, thus allowing the thighs to support the scrotum. In younger children, the scrotum and perineal region should be studied in the “frog leg” position.
The testicles should be examined with multiple-frequency linear array transducers in transverse and longitudinal planes along their entire length. The spermatic cord and its vessels should also be traced along their entire length up to the deep inguinal ring. Depending on the indication and/or US findings, the study should be extended to include the abdominal cavity. Color Doppler and power Doppler must be included in the assessment, with settings adequate to detect low flow velocities.
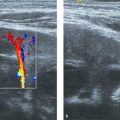
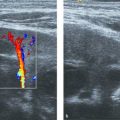
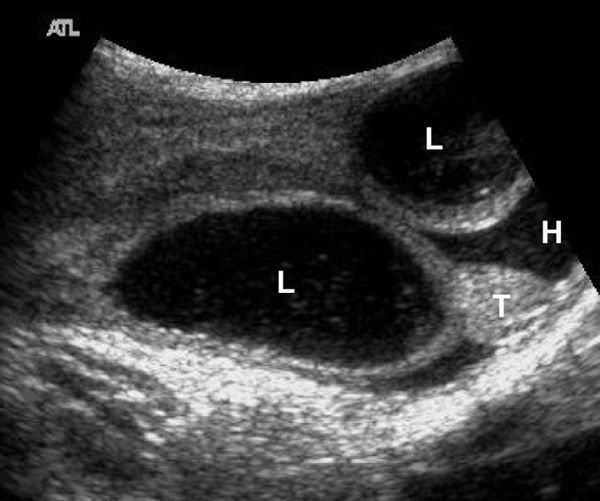
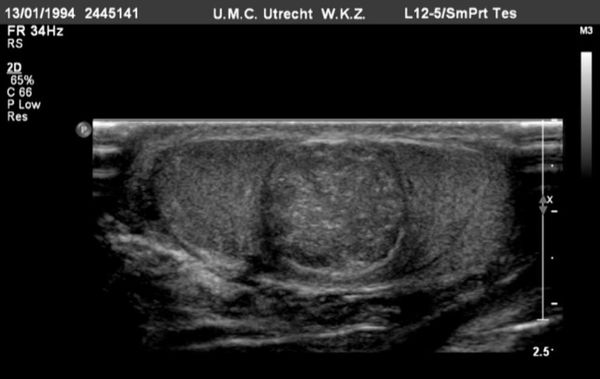
On US, the testis appears as an elliptical and isoechoic organ with a smooth echotexture ( Fig. 16.1 ). The tunica albuginea is seen as a hyperechoic line covering the surface of the testis. The mediastinum presents as a hyperechoic central linear structure crossing the organ ( Fig. 16.1 , Fig. 16.2 , Fig. 16.3 , Fig. 16.4 ). Testicular volume is calculated with the formula V = L × W × H × 0.52, where V = volume, L = length, W = width, and H = height. The volume should be evaluated according to the patient’s age ( Table 16.1 ).
Age | Volume (mL) Mean (SD) |
1 months | 0.30 (0.10) |
3 months | 0.36 (0.10) |
5 months | 0.39 (0.10) |
7 months | 0.32 (0.10) |
9 months | 0.31 (0.10) |
11 months | 0.30 (0.10) |
2 years | 0.31 (0.10) |
6 years | 0.31 (0.10) |
Abbreviation: SD, standard deviation. Source: Created from data within Kuijper EA, van Kooten J, Verbeke JI, van Rooijen, Lambalk CB. Ultrasonographically measured testicular volumes in 0- to 6-year-old boys. Hum Reprod 2008;23(4):792–796, with permission of Oxford University Press. Note: In total, of 344 boys from different ethnic backgrounds were studied. Testicular volume was calculated with the formula length × width × height × 0.523. No differences were found between ethnic groups or between right and left testicles. | |
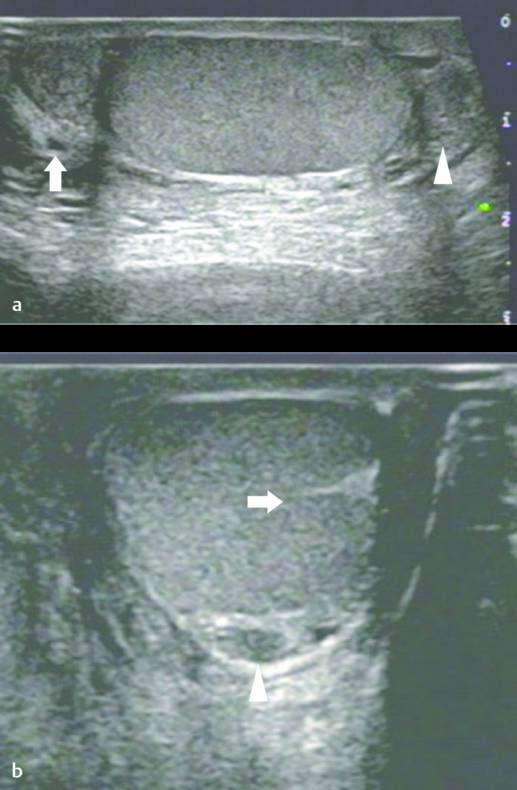
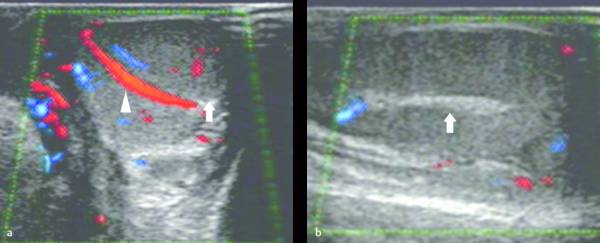
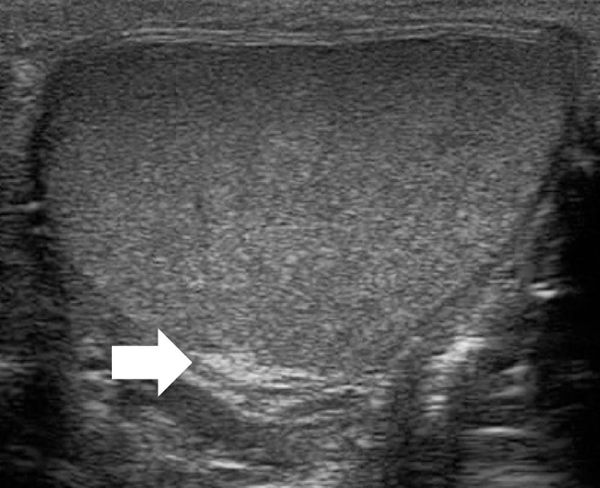
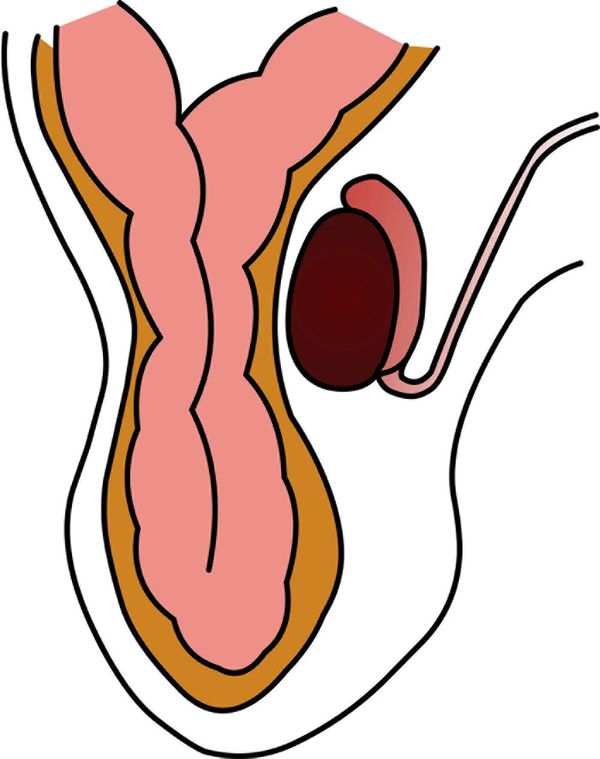
The epididymis appears as an elongated structure with increasing volume from the tail inferiorly to the head superiorly. Its echogenicity is similar to that of the testicle ( Fig. 16.1 and Fig. 16.2 ). Generally, in the transverse plane, the epididymis is located adjacent to the rear aspect or to the side of the testicle.
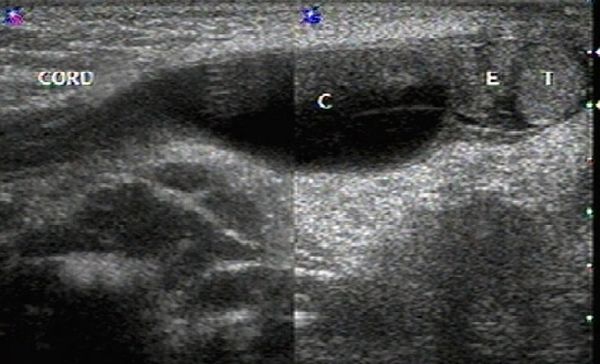
The spermatic cord consists of the vas deferens, which is visible as a hypoechoic tubular structure ( Fig. 16.1 b), together with a network of veins (pampiniform plexus) and arteries.
Tips from the Pro
The US coupling gel should be adequately heated and must be applied in generous amounts.
16.2 Hydrocele and Indirect Inguinal Hernia
16.2.1 Hydrocele
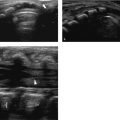
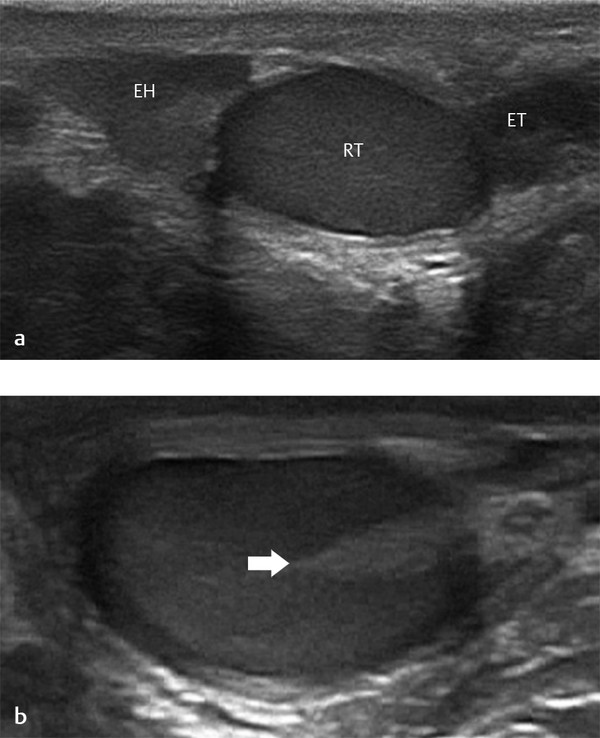
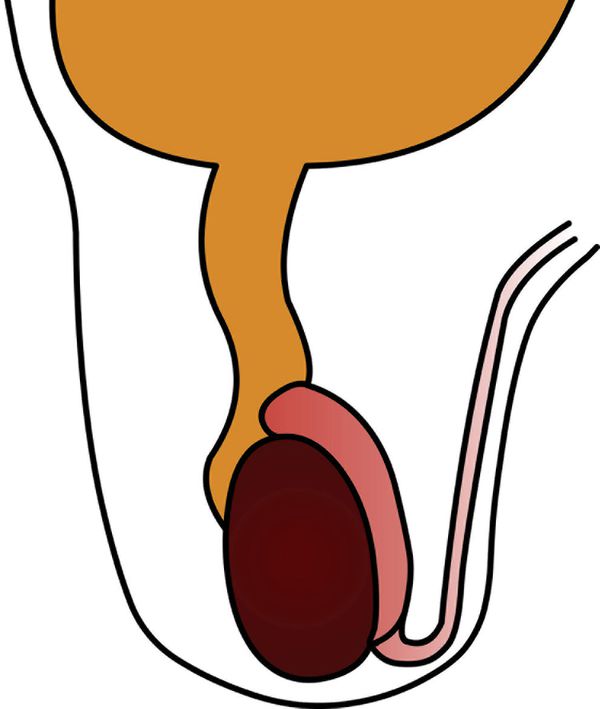
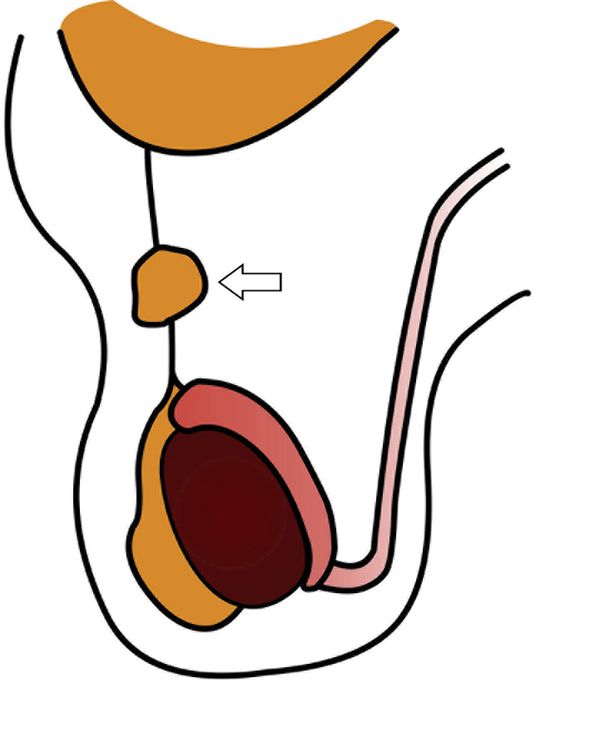
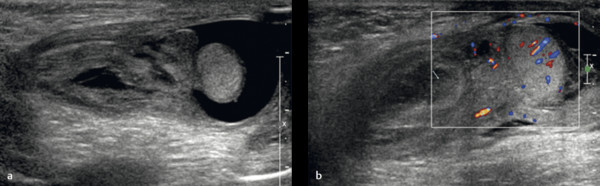
A hydrocele is abnormal fluid in the scrotum. This can be classified as a communicating hydrocele or a noncommunicating hydrocele. The processus vaginalis is patent in communicating hydrocele ( Fig. 16.5 ), whereas it is closed in noncommunicating hydrocele. A communicating hydrocele may be associated with an indirect inguinal hernia ( Fig. 16.6 ). Noncommunicating hydrocele is common in newborns and can be quite large. It occurs as a result of the increased production and malabsorption of scrotal fluid. In older children, noncommunicating hydrocele can be observed after viral diseases or after trauma.
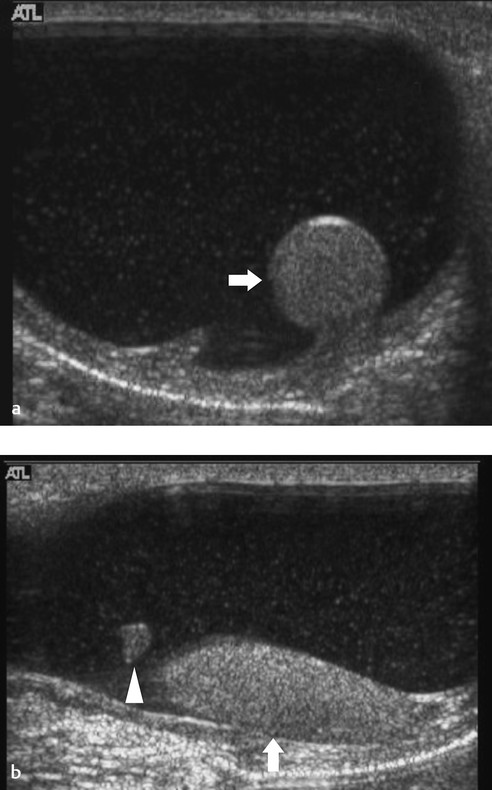
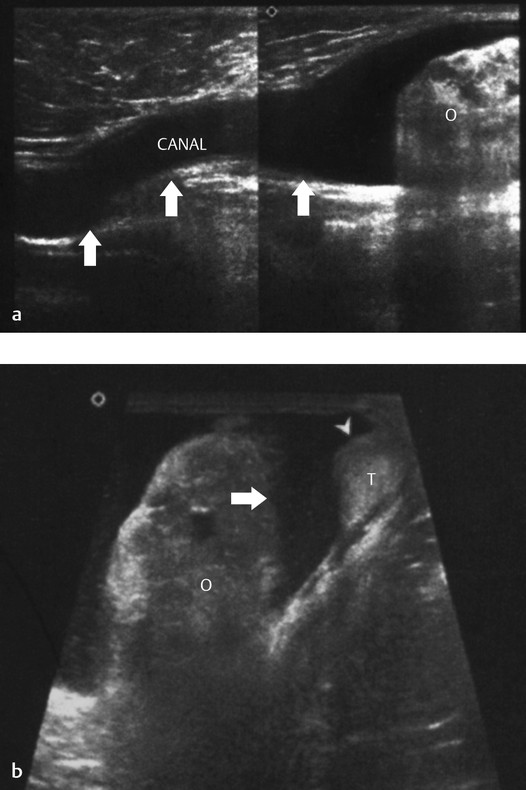
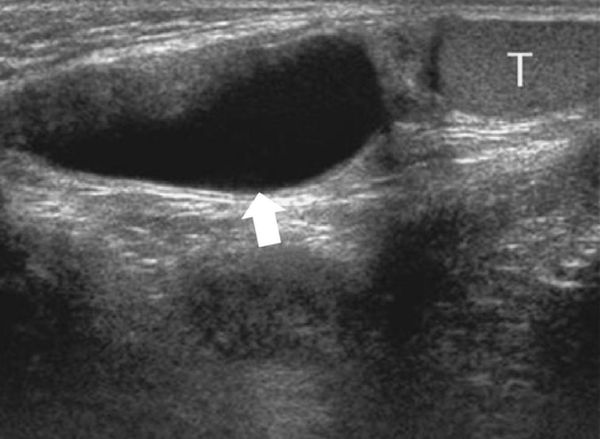
When a clinical diagnosis of hydrocele has been established, US is requested to assess whether a communication with the peritoneal cavity is present and to exclude an associated hernia. On US, a hydrocele is characterized by different quantities of anechoic fluid surrounding the testis. When the hydrocele is small, only a thin layer of fluid is identified along the spermatic cord (Video 16.1). In these cases, gentle manual compression of the scrotum displaces the liquid toward the inguinal canal, allowing determination of the patency of the canal (Videos 16.1 and 16.2). When a hydrocele is massive, it can be challenging to identify the testicle ( Fig. 16.6 and Fig. 16.7 ). It is important to differentiate communicating hydrocele from noncommunicating hydrocele. The communication with the peritoneal cavity is usually easily identified in massive communicating hydrocele. However, the hydrocele may be encysted in the spermatic cord (spermatic cyst; Fig. 16.8 , Fig. 16.9 , Fig. 16.10 ), with quite characteristic appearances.
16.2.2 Indirect Inguinal Hernia
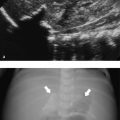
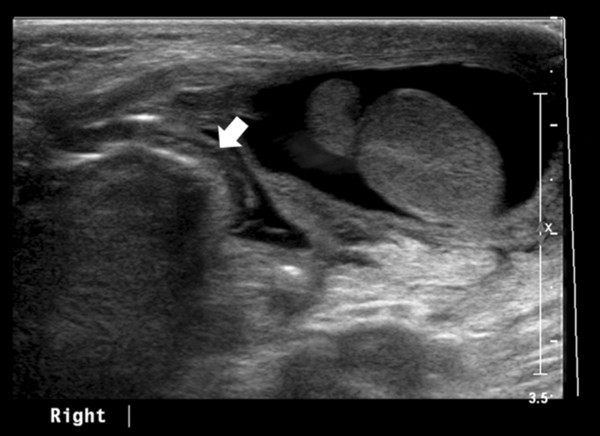
Indirect inguinal hernia occurs when the processus vaginalis remains patent, and consequently, bowel loops, omentum, or mesentery may herniate into the scrotum ( Fig. 16.11 ). Inguinoscrotal hernias are six times more frequent in boys than in girls, and they are more common on the right side and in premature infants.
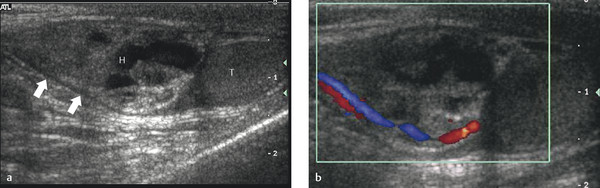
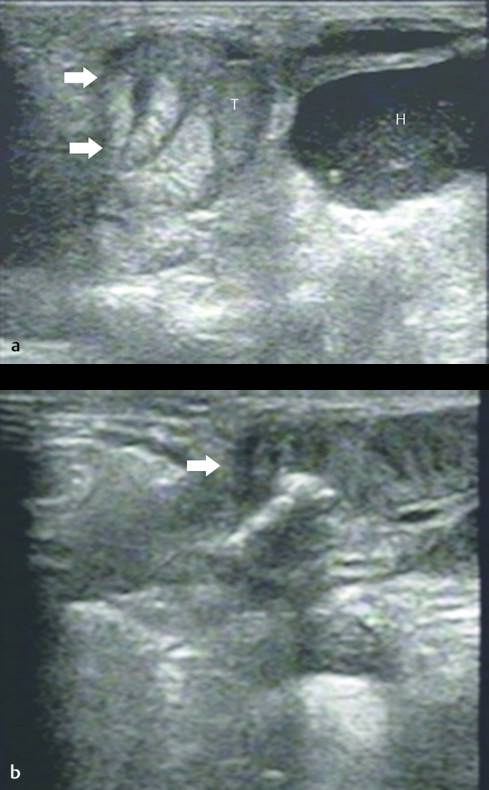
US is useful in the diagnosis of incarcerated scrotal hernias that clinically present as a scrotal “mass.” These irreducible hernias can be classified as obstructive (when the bowel is obstructed), incarcerated (incarcerated by adhesions), or strangulated (when the blood supply of the herniated bowel is compromised).
US has an accuracy of 95% for the detection of a hernia. Omental fatty tissue may present as a hyperechoic tubular structure or mass ( Fig. 16.7 ). The bowel may demonstrate peristalsis and can be identified by its contents: air, liquid, and/or echogenic fecal material ( Fig. 16.12 ; Video 16.12a,b,c Fig. 16.13 , Fig. 16.14 ; Video 16.3). When bowel is identified, the assessment of peristalsis is important. The absence of peristalsis may be related to a nonviable bowel. The presence of an akinetic bowel loop inside the scrotum has a sensitivity of 90% and a specificity of 100% for the diagnosis of strangulation ( Fig. 16.15 ).
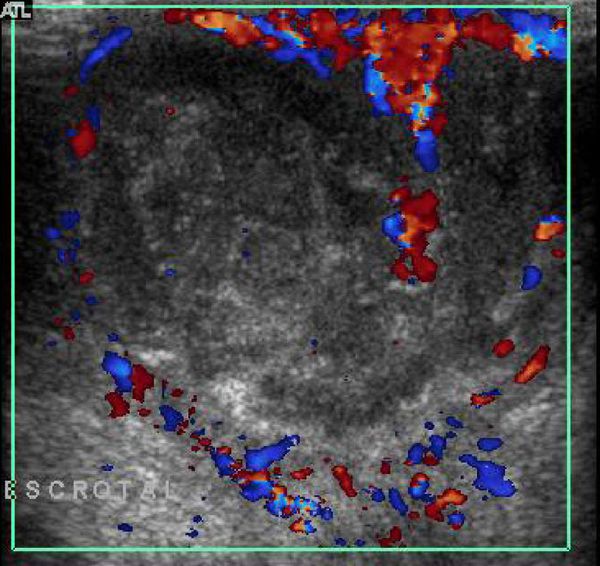
Sometimes, the groin is swollen in the immediate postoperative period. The radiologist can be asked to exclude an acute recurrence of the hernia. Most of the time, the swelling is caused by edema and/or hematoma ( Fig. 16.16 ). Rarely, the hernia persists or recurs after herniotomy. One should look for a bowel loop in the groin that can be followed toward the intraperitoneal bowel. It will often demonstrate peristalsis ( Fig. 16.17 ).
Postoperative complications of inguinal hernia repair include seroma, hematoma, abscess, and displacement or “foldings” of the prosthetic mesh. On US, edema and thickening of the subcutaneous tissues of the inguinal region are seen. Seroma, abscess, and hematoma appear as fluid collections with mixed echogenicity ( Fig. 16.15 , Fig. 16.16 , Fig. 16.17 ). A prosthetic mesh appears as a linear, hyperechoic structure measuring about 2 mm in thickness, with a fine irregular surface and posterior acoustic shadowing. The position and disposal of the prosthetic mesh can also be evaluated during the scan.
Following inguinal hernia repair, the patient should be monitored for testicular atrophy as a result of venous or arterial injury/obstruction in the spermatic cord. A hernia may recur in up 17% of operated patients.
Tips from the Pro
The best method to investigate a potential inguinal hernia is to ask the child to cough while standing, or to assess the area while the child is crying.
16.3 Scrotal Tumors
16.3.1 Testicular Tumors
Testicular tumors are rare in boys, accounting for 1 to 2% of all pediatric solid tumors. Malignant germ cell tumors, like seminoma and embryonal carcinoma, are rarely present in prepubertal patients. Teratoma is the most common testicular tumor in children and accounts for nearly 50% of prepubertal testicular tumors. Teratomas are mostly benign. They are formed of different germ cells of endoderm, mesoderm, and ectoderm. The average age of patients at presentation is 18 months, but these tumors can occur in the neonatal period. They can cause a rise in the serum alpha1 fetoprotein level.
Gonadal stromal cell tumors account for 13% of the total; they include granulosa cell (5%), Leydig cell (4%), Sertoli cell (3%), and mixed gonadal stromal cell (1%) tumors.
Epidermoid cysts constitute approximately 1% of testicular tumors. They are more common in nonwhite males in the second or third decade of life at the time of diagnosis. Epidermoid cysts are benign intratesticular masses, and unlike mature teratomas, they have no malignant potential. They are composed of keratinizing, stratified, squamous epithelium with a well-defined fibrous wall.
Testicular microlithiasis is found in 0.6% of patients undergoing US but is present in about 50% of men with a germ cell tumor. It is much more common in patients without cancer. A meta-analysis of adult studies showed that in men without other risk factors for testicular cancer, the presence of testicular microlithiasis should not lead to further investigations or follow-up studies.
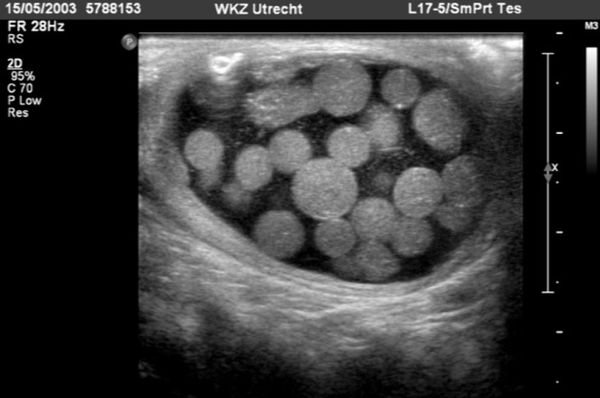
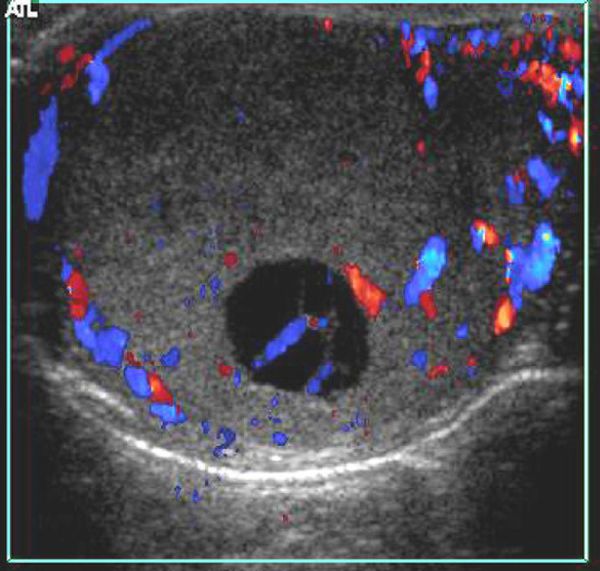
Testicular tumors have very different US appearances; these lesions can be cystic, cystic–solid, or solid, and they may contain calcifications ( Fig. 16.18 and Fig. 16.19 ; Video 16.19). Although epidermoid cysts are true cysts, they appear solid on radiologic images. They appear as well-circumscribed, round to slightly oval hypoechoic nodules, which may have laminations. The laminations often give rise to an “onion skin” or ringed appearance, and the nodule may have a hyperechoic wall that is sometimes calcified ( Fig. 16.20 ). Gonadal stromal tumors may present as well-defined hypoechoic nodules ( Fig. 16.21 ). These tumors can be quite large at diagnosis, affecting the entire testicle with associated cystic areas. Yolk sac tumors and choriocarcinomas have variable US presentations; they may be homogeneous or heterogeneous and may contain cystic areas of necrosis and/or calcifications ( Fig. 16.22 ). On US, microlithiasis appears as small nonshadowing hyperechoic foci ranging in diameter from 1 to 3 mm. Testicular microlithiasis is often bilateral ( Fig. 16.23 ).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree