2 Cryoablation: Mechanism of Action and Devices
Though initially introduced in the 19th century, cryoablation entered mainstream clinical medicine in the mid-1960s.1 Because of technical limitations, however (i.e., large-bore probes to accommodate the large gas flow required and to facilitate adequate heat exchange), its applications were limited in the operative setting. In the last few years, as technology spawned the introduction of thinner cryoprobes (down to 14-gauge), image-guided, percutaneous cryoablations have carved out an ever-expanding niche. Currently, cryoablation is extensively used in the treatment of prostate cancer and renal cancer (either during open surgery or laparoscopically). Image-guided percutaneous cryoablation is mostly used for the treatment of small renal cancers and for the palliation of painful bone lesions. To a lesser extent, it is also used for the treatment of primary and secondary liver and chest neoplasms.
The technical goal of cryoablation is to reduce the temperature of the target tissue as well as an appropriate surrounding margin (equivalent to the surgical margin) to below lethal levels, without damaging vital nearby organs. For mammalian tissue, lethal temperature is below approximately –20° to −25°C (or –4° to –13°F). Cytotoxicity is mediated via various mechanisms, including ischemia, denaturing of protein structures, and the breakdown of cellular and intracellular membranes due to the formation of ice crystals. Some authors believe that the extracellular scaffolding structure is relatively preserved during cryoablation. This theoretically allows for the cellular repopulation of the tissue, which may explain why cryoablation is more forgiving, compared with radiofrequency ablation (RFA), in cases of nontarget ablation.
♦ The Physics of Cryoablation
The objective of cryoablation (kryo = “cold” in Greek; and ablatus = “to carry away” in Latin) is to reduce the temperature of the target tissue to below the lethal −20°C. This requires a continuous removal of energy from the entire target tissue. There are three ways that thermal energy may be transferred from one volume to another: (1) by conduction, the transfer of energy (or heat) from an object to a another at a lower temperature by virtue of physical conduct (direct molecular kinetic energy exchanges); (2) by convection, the transfer of energy (or heat) from an object to another at a lower temperature, with one of the objects being a flowing fluid (thus providing a continuously refreshed heat sink or heat source); and (3) by radiation, which removes radiant energy from all bodies above absolute zero temperature. No significant radiation losses are observed in our case; therefore, we can concentrate on conduction and convection ( Fig. 2.1 ).
Conduction is a steady-state transfer because (in our case) it depends only on the temperature difference, which is relatively stable (i.e., tissue temperature [37°C] versus probe temperature [approximately −140°C]). On the other hand, convection depends on the flow rate of the blood through or adjacent to the volume of interest. Therefore, large-diameter arteries or very vascular tissues, to a lesser degree, will limit the extent of cryoablation. The reverse of this phenomenon (cooling of tissue by nearby flowing blood) has been termed “heat sink” in the case of thermal ablations (such as RFA or microwave ablation).
One advantage of cryoablation over RFA and microwave ablation is that the tissue energy transfer characteristics do not appreciably change with temperature. In contrast, in the case of RFA, tissue desiccates at high temperature and no further transfer of energy is possible. Irrespective of the tissue temperature, further temperature reduction is always theoretically possible with cryoablation.
The removal of energy (cooling) from the tissue surrounding the cryoprobe is effected by the Joule-Thomson law.2 During gas expansion two opposing mechanisms take effect. First, as intermolecular distances increase with cooling, the potential energy stored between molecules increases. Because total energy must be conserved, this translates into lower molecular kinetic energy and therefore lower temperature. Second, as intermolecular distance increases, however, fewer molecular collisions result in a relative decrease in potential energy stored between molecules. Again, because total energy must be conserved, this results in greater molecular kinetic energy and therefore greater temperature. Which of the two competing mechanisms dominates depends on the so-called inversion temperature of the gas, which is an intrinsic property. For argon, nitrogen, or oxygen, the inversion temperature factor is greater than room (or ambient) temperature and the first mechanism dominates. For helium and hydrogen, the inversion temperature is less than room temperature. Therefore, expansion of compressed argon, nitrogen, or oxygen causes cooling, whereas expansion of helium or hydrogen causes warming.

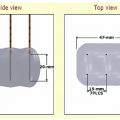
The gas is supplied by a gas tank to the probe via a pressure regulator ( Fig. 2.2 ). Gas expansion occurs within the dual chambered cryoprobe ( Fig. 2.3 ). The gas, once it expands, cools, absorbs thermal energy from the surrounding tissues, and is siphoned back through the tubing and eventually released into the atmosphere. The diameter of the “ice ball” depends on the rate of energy transfer, which in turn depends on the flow rate of the cooling gas through the probe. The length of the ice ball mostly depends on the uninsulated length of the probe ( Fig. 2.4 ).
It is worth repeating that the lethal temperature for mammalian tissue is −20°C or colder. This does not correspond to the visible margin of the ice ball as seen under computed tomography (CT), ultrasound (US), or magnetic resonance (MR) guidance, which is at 0°C. Studies have shown that the −20°C isotherm is at least 5 mm inside the 0°C isotherm.3
♦ Ablation Systems
There are currently two U.S. Food and Drug Administration– approved cryoablation systems available in the United States, which at the time of writing of this chapter were in advanced merger discussions. Both systems, Endocare and Galil, make use of the above basic physical properties of the gases, and both utilize argon for freezing and helium for thawing. This may change in the near future as technologic advancements may allow the reintroduction of a single gas cooling-thawing system using nitrogen.



Endocare
With the Endocare (Irvine, CA) system, there are four cryoprobe types available for use, differing in the shape and size of the generated ice ball ( Fig. 2.5 ). Probe selection depends on the size and shape of the target lesion. For example, a 1-cm lesion in a difficult-to-access location is best approached with the Perc-15 probe. In general the Perc-24 is the workhorse probe for this system, as it yields the largest ablation zone. The respective isotherms for each probe are graphically shown in Fig. 2.6 . Endocare also provides a percutaneously inserted temperature sensor ( Fig. 2.5 ). This may be useful in rare instances when the temperature near a nontarget organ needs to be monitored.
The Endocare regulator is shown in Fig. 2.7 . The regulator is composed of gas inlet hookups (argon and helium in); gas outlet hookups (argon, helium out); the internal regulator design; the control panel; and the screen, which can display the readings from up to eight cryoprobes and eight temperature sensors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree