Abstract
The 22q11.2 deletion syndrome is the most common human chromosomal microdeletion syndrome and one of the most common genetic syndromes associated with prenatally detected congenital heart defects (CHDs). It has a wide range of associated clinical findings. Its more severe presentation, DiGeorge syndrome, presents with thymus aplasia and severe immunologic abnormalities along with other developmental defects. Fluorescence in situ hybridization (FISH) and the now more widely used chromosomal microarray analysis (CMA) reliably detect the common 1.5 to 3 Mb deletions in children with features of 22q11.2DS, and prenatally in the fetus through amniocentesis or chorionic villus sampling (CVS). Recently, noninvasive prenatal screening for microdeletion syndromes from cell-free fetal DNA (cffDNA) in maternal plasma has been introduced in the clinic with variable false positive and false negative rates. This is changing prenatal ascertainment of 22q11.2DS from specific targeted diagnosis based on identified fetal anomalies, such as a CHD, to a broader screen for fetuses with other anomalies or for apparently unaffected pregnancies. Considering the variability and severity of its clinical features, it is important that practitioners know the prenatally detectable typical and atypical findings of 22q11.2DS, as well as the health issues and complications of the syndrome that are not prenatally observable and the implications for prenatal management and counseling when 22q11.2DS is diagnosed in a pregnancy.
Keywords
22q11.2 deletion, congenital heart disease, thymus aplasia, chromosomal microarray, other anomalies
Introduction
The 22q11.2 deletion syndrome is the most common human chromosomal microdeletion syndrome and one of the most common syndromes associated with prenatally detected congenital heart defects (CHDs). It has a variable phenotype, with a wide range of associated clinical findings. DiGeorge syndrome, first described in 1965, is its most severe presentation, with primary symptoms of thymus and parathyroid aplasia and severe immunologic abnormalities; CHDs were a later added feature. Other names, based on presence of predominant features, have been given to the syndrome and include Shprintzen or velocardiofacial syndrome, autosomal dominant Opitz G/BBB syndrome, Caylor cardiofacial syndrome, and CATCH 22 ( c ardiac abnormality, a bnormal facies, t hymic hypoplasia, c left palate, h ypocalcemia from deletion in chromosome 22). Considering the recognition that most affected individuals with these conditions have a deletion in 22q11.2, the recommended name is now 22q11.2 deletion syndrome (22q11.2DS). Fluorescence in situ hybridization (FISH) and the more recently preferred chromosomal microarray analysis (CMA) reliably detect the most common 1.5 to 3 Mb deletions in children with features of 22q11.2DS, and prenatally in the fetus through amniocentesis or chorionic villus sampling (CVS). More recently, noninvasive prenatal screening for microdeletion syndromes from cell-free fetal DNA (cffDNA) in maternal plasma has been introduced in the clinic with variable false positive and false negative rates and is changing prenatal diagnosis of 22q11.2DS from a specific targeted test offered because of specific identified fetal anomalies, such as CHDs, to a broader screen for apparently unaffected pregnancies, and occasionally for fetuses with other abnormalities. Considering the variability and severity of the clinical features of 22q11.2DS, it is important that practitioners know its prenatally detectable findings, health issues, and complications of the syndrome that are not prenatally observable, and implications for prenatal management and counseling when it is diagnosed in a pregnancy.
Disorder
Definition
The 22q11.2DS is a pleiotropic genomic disorder with a widely variable clinical presentation, caused by heterozygous deletions of 0.7 Mb to 3 Mb in the q11.2 region of chromosome 22. It can present with varying combinations of features listed in order of decreasing frequency in Table 154.1 . Currently, 22q11.2DS has replaced other nomenclature, and the term DiGeorge syndrome is reserved for the more severe form associated with thymic hypoplasia or aplasia and hypocalcemia. Velocardiofacial syndrome, which points to the typical combination of velopharyngeal incompetence leading to hypernasal speech, cardiac abnormality, and distinct facial features seen in many individuals with 22q11.2DS, is now less commonly used to describe this microdeletion syndrome.
| Feature | Likelihood in Affected Individuals (%) |
|---|---|
| Characteristic facial features | >80 |
| Congenital cardiac defects | 75 |
| Immune deficiency, including thymic hypoplasia | 77 |
| Palatal abnormalities | 69 |
| Feeding, swallowing, and gastrointestinal problems | 30 |
| Learning difficulties | 70–90 |
| Hypotonic limbs and hyperextensible joints | 60 |
| Hypocalcemia and hypoparathyroidism | 50 |
| Microcephaly | 50 |
| Hearing loss | 40 |
| Genitourinary anomalies | 31 |
| Neuropsychiatric disease | 25 |
Prevalence and Epidemiology
It is estimated that 22q11.2DS affects 1 : 3000 to 1 : 6000 individuals, making it the most prevalent microdeletion syndrome. Although there is no known predisposition based on ethnicity or gender, data from the United States Centers for Disease Control and Prevention suggest that Hispanics have a higher prevalence of 22q11.2DS than non-Hispanic Americans (1 : 3800 versus 1 : 6000). It is the most common cause of syndromic abnormality of the palate and the second most common cause of CHDs with developmental disability. Although there is increased early death, with modern treatments, 90% to 95% of individuals survive beyond infancy. Some recent reports of more widely performed prenatal testing and screening suggest that the incidence is higher (approx. 1 : 350 to approx. 1 : 1000) in prenatally tested pregnancies, and close to 1 : 100 in fetuses with CHDs and other major structural anomalies. This may imply that some prenatally detected affected fetuses could either demise prior to delivery or postnatally before a genetic diagnosis is made. Alternatively, milder or asymptomatic cases may be more common but until now were undetected. Considering the wide clinical variability and the fact that subtle cardiac defects may remain undiagnosed for prolonged periods, it is possible that the true prevalence of 22q11.2DS is underestimated. This is consistent with reported higher incidence data of 1 : 1000 from noninvasive cell-free DNA screening and the clinical observation that mildly affected individuals can be unrecognized until they have a more severely affected child.
Etiology, Pathophysiology, and Embryology
The developmental abnormalities in 22q11.2DS involve primarily craniofacial structures, thymus, hypoparathyroid, and cardiac outflow structures, and can be explained by abnormal development of mesoderm, endoderm, and neural crest derivatives of the branchial arches. The most typical cardiac defects are a type B interrupted aortic arch, which results from aplasia of the left fourth pharyngeal artery, and tetralogy of Fallot, caused by defective development of the pulmonary infundibulum.
The 22q11.2DS is a heterozygous contiguous deletion syndrome that is usually sporadic and de novo (90%–95%) but may be inherited in an autosomal dominant fashion in approximately 10% of cases. There is a 50% risk to transmit the deletion from an affected parent, but clinical severity in the offspring is variable. De novo deletions occur more commonly on the maternally inherited chromosome 22. Because of potential gonadal mosaicism for the deletion, there is approximately 1% risk for recurrence, even in apparent de novo cases. The genomic locus contains many low-copy-number repeat (LCR) sequences of DNA that predispose to the formation of duplications and deletions in this region during meiosis. The most common deletion (in 85% of cases) is 3 Mb, but up to 10% have a smaller 1.5 Mb deletion in the proximal half of the region, followed by smaller and also more distal deletions of 0.7 Mb to 1.5 Mb. There are 90 genes in the 3 Mb deletion, including protein-coding genes, microRNAs and long noncoding RNAs, not all of which cause disease when one copy is missing.
Human studies and mouse models have identified that TBX1 is a critical gene for many of the features, in particular the heart defects, but other genes, such as CRKL, may be involved in smaller deletions that do not include TBX1. Among the genes that have been linked to the cognitive deficits and neuropsychiatric symptoms are COMT, PRODH, and DGCR8. Although most individuals with the syndrome have 22q11.2 deletions, some have point mutations in TBX1, or have no clearly identified genetic cause. Other chromosomal defects have been described, mostly deletions of 10p responsible for DiGeorge syndrome/velocardiofacial syndrome complex 2.
Manifestations of Disease
Clinical Presentation
Most prenatally diagnosed cases of 22q11.2DS syndrome are identified because of abnormal cardiac anatomy during routine screening prenatal ultrasound (US), but more recently they may be ascertained through genetic testing by CMA or noninvasive cffDNA screening. Of the approximate 10% of affected fetuses with an inherited 3 Mb deletion, the affected parent can be asymptomatic or have conditions, such as neuropsychiatric disease, not previously recognized to be caused by the deletion. This number may be slightly higher for smaller atypical deletions. This variability underscores the importance of a thorough prenatal US exam at 18 to 20 weeks’ gestation, including imaging of the four-chamber heart, three vessels, and cardiac outflow tracts crossing and long-axis views, for optimal detection of CHDs and identification of at-risk fetuses.
When a CHD, in particular a conotruncal defect, is detected prenatally, 22q11.2DS should always be considered. Postnatally, in addition to (or sometimes in absence of) a CHD, velopharyngeal incompetence with palatal abnormalities (bifid uvula and palatal submucosal clefts) with feeding and speech difficulty, learning disability and psychopathology, hypocalcemia secondary to hypoparathyroidism, T-cell immune defects secondary to thymic hypoplasia, typical facial features (square nasal root, bulbous nose with hypoplastic alae nasi, small palpebral fissures, elongated face, smooth philtrum, simple protruding and low-set ears, and tapered fingers), and hearing loss all can be found with variable frequency.
Structural abnormalities of other organs (genitourinary, central nervous system [CNS], skeletal, gastrointestinal, laryngotracheal) are less commonly described, but can certainly be present and be diagnosed prenatally.
Imaging Technique and Findings
Ultrasound.
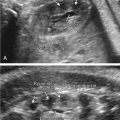
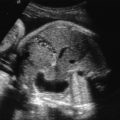
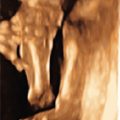
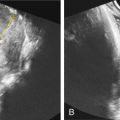
With the increased screening and testing, often before the first anatomical survey US is performed; accurate recognition of potentially visible clinical features and knowledge of their frequency in 22q11.2DS is critical. About 75% of affected fetuses and infants have CHDs, typically conotruncal anomalies, but other defects can be present together with those or in isolation. The most common are tetralogy of Fallot (20%) ( Fig. 154.1 ), interrupted aortic arch (seen in 13%; in patients with type B interrupted aortic arch, 52% have 22q11.2DS) ( Fig. 154.2 ) or hypoplastic aortic arch ( Fig. 154.3 ), ventricular septal defect (14%) with or without pulmonary atresia, truncus arteriosus (6%) ( Fig. 154.4 ), vascular ring (5.5%) as well other defects (10%) such as hypoplastic left heart ( Fig. 154.5 ), endocardial cushion defect ( Fig. 154.6 ), or atrial septum defects.



Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree