Diagnosis: Pulmonary embolism
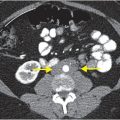
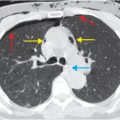
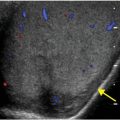
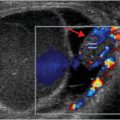
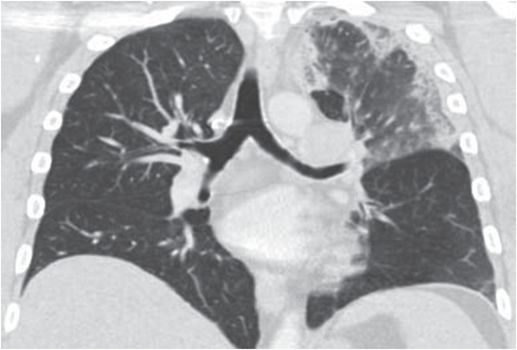
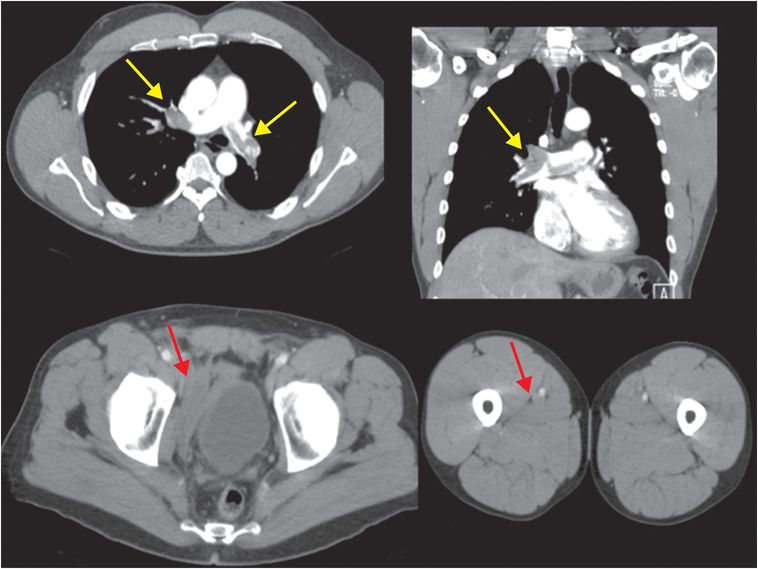
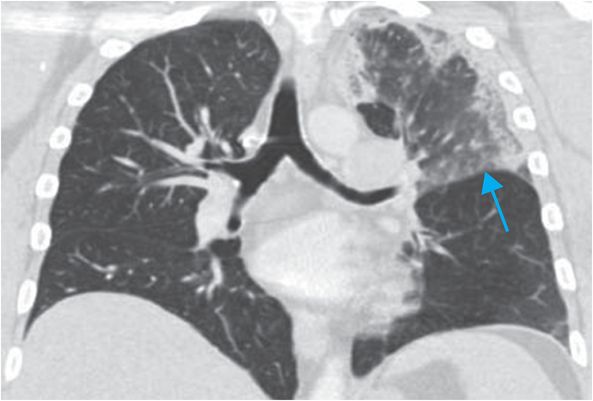
Axial and coronal images from a CT pulmonary angiogram including the thighs and pelvis demonstrate central filling defects in the right and left main pulmonary arteries (yellow arrows). There is hypodense thrombus expanding the right external iliac and femoral veins (red arrows). Coronal CT in lung window shows a wedge-shaped, peripheral infarct (blue arrow).
Discussion
Pulmonary embolism (PE) is the third most common cause of death from cardiovascular disease after myocardial infarction and stroke, and is a leading cause of preventable morbidity and mortality in hospitalized patients.
Estimates of incidence of venous thromboembolism (VTE, defined as PE and deep venous thrombosis, or DVT) range from 300,000 to 600,000 per year.
Two-thirds of patients with VTE present with DVT, while one-third present with PE.
While some patients are asymptomatic, up to 25% of patients with PE may have a first presentation of sudden cardiac death.
Establishing a diagnosis of VTE may be challenging given variation in clinical presentation and variable sensitivity and specificity of diagnostic modalities. VTE may be asymptomatic or have subtle clinical manifestations in some cases. History, physical exam, laboratory studies, and imaging play vital and complementary roles.
Risk factors include inherited thrombophilias, pregnancy, malignancy, obesity, chronic disease, advanced age, iatrogenic factors (hormonal therapies, hospitalization, trauma, and surgery), and immobilization.
Typical clinical findings in DVT include lower extremity edema, warmth, and erythema. PE tends to present with dyspnea, pleuritic chest pain, tachypnea, syncope, and cough.
Several clinical probability-scoring systems have been developed – the Wells criteria, with separate systems for determining the probability of DVT and PE, is perhaps the most well known (the Geneva score is also well validated). Based on the presence of risk factors, signs, and symptoms, patients are stratified into low-, intermediate-, and high-probability categories.
Testing for elevated D-dimer, a fibrin degradation product, is sensitive but not specific for VTE – the negative predictive value is approximately 94%; however, D-dimer may be elevated in other conditions, including renal impairment, pregnancy, and hemorrhage. Thus, the D-dimer assay is used in conjunction with prediction rules such as the Wells criteria to determine the need for further work up including imaging.
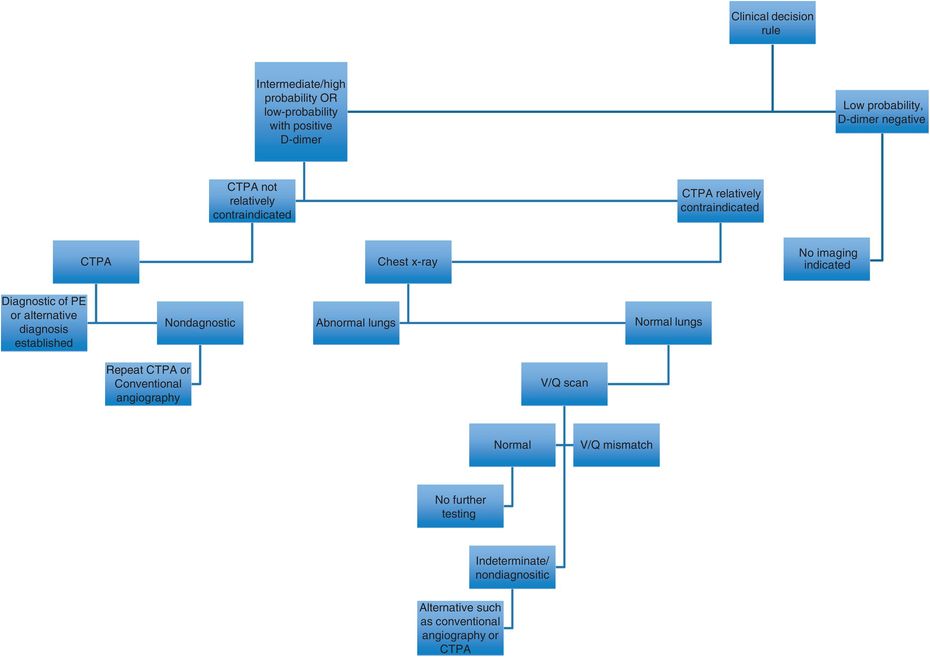
Imaging modalities used in the work-up of PE include lower extremity ultrasound to assess for DVT (up to 35% of patients with DVT above the knee have silent PE), nuclear medicine ventilation-perfusion (V/Q) scanning, conventional angiography, and CT pulmonary angiography (CTPA), which is the most commonly used imaging modality in the United States.
Choice of imaging modality is dependent on multiple clinical factors. American College of Radiology (ACR) appropriateness criteria and other guidelines are available to guide modality selection in cases of suspected PE.
CTPA should be used in patients with low pretest probability for PE and an abnormal D-dimer result, or in those who have a high pretest probability irrespective of D-dimer result. Clinical validity for CTPA in diagnosing PE is similar to that of conventional pulmonary angiography and V/Q scanning. Strengths of CTPA over other modalities include non-invasiveness, wide availability, convenience, and ability to identify alternative diagnoses. Drawbacks of CTPA include risk of contrast nephropathy and exposure to ionizing radiation.
Currently, conventional angiography is reserved for cases in which clinical suspicion remains high in the setting of negative CTPA or when intervention is being considered, as in hemodynamically significant PE.
V/Q scanning is useful in select situations, but is non-diagnostic in many cases and, therefore, usually second-line in the emergency setting.
In pregnant patients, some guidelines indicate that a chest x-ray to evaluate for alternative diagnoses is the preferred initial approach. If normal, the next indicated study is a perfusion (Q) scan. This is only followed by a ventilation (V) scan to assess for V/Q mismatch indicative of PE if the perfusion scan is abnormal (it should be noted, however, that evidence is limited and these approaches are not incontrovertible).
Signs of acute PE differ from those of chronic PE, and management decisions may depend on whether PE is acute or chronic.
Signs of acute PE on CTPA include intraluminal filling defects, visibility of thrombus on three or more images, enlarged artery, mosaic perfusion (abnormal lucent areas due to low local perfusion), and associated hemorrhage, infarction, or cavitation. Technical quality of the CTPA study is important to consider; this includes assessment of adequacy of the contrast bolus and whether there is limiting respiratory motion.
Chronic pulmonary emboli, which can cause secondary pulmonary hypertension and cor pulmonale, tend to be eccentric within the vessel, with webs, calcification, or post-stenotic dilatation. Signs of secondary pulmonary hypertension caused by chronic PE include prominent right ventricle (RV), diameter of right main pulmonary artery >18 mm, diameter of the pulmonary trunk >29 mm, mosaic attenuation, and vascular pruning.
Enlargement of the RV relative to the left ventricle (RV/LV ratio >0.8–1.0), bowing of the interatrial and interventricular septa, reflux of contrast into the inferior vena cava (IVC) and hepatic veins, or engorgement of the superior vena cava and azygos veins should raise concern for right heart strain; patients with this finding should be referred for echocardiography.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree