Introduction
The most frequent orthopedic procedures are everyday fracture reductions, immobilizations, and fixations. The next most important and frequently performed orthopedic procedures are joint arthroplasties, in which a portion of a joint or the entire joint is replaced by a surgically placed prosthesis. The American Academy of Orthopedic Surgeons estimates in 2011 almost 1 million total joint replacements were performed in the United States (Table 4.1).
| Procedure | Hospital discharges |
|---|---|
| Total knee replacement (TKR or TKA) | 645,062 |
| Total hip replacement (THR or THA) | 306,600 |
| Partial hip replacement | 105,506 |
| Total shoulder replacement (TSR or TSA) | 29,414 |
| Partial shoulder replacement | 15,860 |
| Spinal fusion | 465,070 |
A number of overlapping terms are used when discussing joint replacement. Arthroplasty is a generic term for any joint replacement surgery designed to restore joint function. A prosthetic device (prosthesis or implant) may replace the native joint totally or partially. A total arthroplasty involves prosthetic replacement of both sides of a joint, whereas hemiarthroplasty (or hemi-arthroplasty) involves replacement of only one side of a joint. The term prosthesis is a loosely used term for an artificial substitute for a missing body part. Often, the terms prosthesis, implant, and medical device are used interchangeably.
The major indication for joint replacement is advanced degenerative osteoarthritis from everyday wear and tear and/or associated joint-related trauma earlier in life. Inflammatory arthropathy, particularly rheumatoid arthritis, is a common indication for replacement of the elbow and the small joints in the hand and wrist. Infectious arthritis of any joint may cause severe joint destruction warranting joint replacement. Osteonecrosis with destruction of the femoral head or the humeral head is sometimes an indication for joint arthroplasty. Comminuted fractures which cannot be surgically fixed may also lead to joint replacement.
A somewhat rarer indication for joint replacement is limb-sparing surgery (LSS), which is a complex operative procedure most commonly performed by oncology orthopedic surgeons for treatment of extremity sarcomas. The tumor is removed by an extensive excision, and the removed tissue is replaced with a metallic prosthesis, allograft bone, or a combination of an allograft bone metallic prosthesis. The specially designed limb-sparing prosthesis is fixed to the remaining bones with bone cement or press-fit into the remaining bones for later bony ingrowth. Patients with serious accidents or chronic infections sometimes may be candidates for limb-sparing surgery.
The most common joints requiring replacement are the hips, total hip arthroplasty (THA), and knees, total knee arthroplasty (TKA), although total shoulder arthroplasty (TSA) is also common. Joint replacement in the elbow, wrist, hand, ankle, and feet is far less common.
The major contraindications for any joint arthroplasty are systemic infection or localized infection in the joint in question. A n europathic joint is also a contraindication for joint arthroplasty. Joint radiography is nearly always required for preoperative evaluation of a joint being considered for replacement. Cross-sectional imaging – CT, MRI, and sometimes nuclear medicine imaging – may add additional information concerning a joint being evaluated for replacement.
Postoperative evaluation of joint arthroplasty is always initially performed with standard radiography. Cross-sectional imaging with CT or MRI may be used for additional information. The metallic constituents in most joint replacements cause a variety of artifacts on CT and MRI, but there are metal-reduction imaging algorithms which permit very useful cross-sectional imaging of joint arthroplasties (Dula et al., 2014; Fritz et al., 2014; Jansen et al., 2014; Koff et al., 2014). While these algorithms may reduce many artifacts associated with metal implants, they can introduce other artifacts, which may sometimes obscure pelvic structures (Han et al., 2014). For more information, see Chapter 8.
It is important to recognize the proper positioning of an implant as well as the complications that may occur. The specific names of the numerous arthroplasty designs are not important per se and should not be mentioned in the radiology report, unless one is 100% sure about the name. Implants are often described as being constrained or non-constrained (also called unconstrained). Constraint is the resistance of an implant to a particular degree of motion in a given plane, most often anterior–posterior translation or axial rotation. An implant may be described as fully constrained (very limited motion in a given direction), semiconstrained (intermediate amount of motion in a given direction), or non-constrained (allowing full motion in a given direction).
Conformity is the geometric measure of the articulation fit between the joint components – fully conforming prostheses have full articular contact. The greater the conformity, the larger the contact area between components, and the less intrinsic stress wear. Modularity is the ability to add implant components, such as stems, augments, and wedges, to enable the orthopedic surgeon to make a custom prosthesis intraoperatively (Barrack, 1994).
A custom prosthesis is a preoperatively designed implant having features to accommodate the specific needs of the patient. Fixation of a joint implant to the skeleton is accomplished in a variety of ways using press-fit stems, cerclage wires, fixation screws, and cementing with polymethylmethacrylate (PMMA). As a general rule, bone cement used with joint arthroplasty increases component fixation and is frequently used. Bone cement has its downsides as well. It may damage the surrounding bone and leak into anatomic spaces, where it can produce neurovascular injury. It also makes any needed subsequent revision arthroplasty considerably more difficult.
Shoulder Arthroplasty
Shoulder arthroplasty was first tried in 1893 by Jules-Emile Pean, a French surgeon who implanted a platinum and rubber prosthesis to replace a shoulder joint destroyed by tuberculosis (Schrumpf et al., 2011). The modern era for shoulder replacement began with the work of Charles Neer from 1951 to 1974, when the first modern total shoulder prosthesis, the Neer II, was introduced. This consists of a metallic humeral head and stem component which articulates with a polyethylene glenoid resurfacing component. Since then, over 70 different shoulder prosthetic devices have been developed (Schrumpf et al., 2011).
The main indications for shoulder arthroplasty are severe shoulder degenerative or inflammatory arthritis, humeral head osteonecrosis, or a complex, highly comminuted proximal humerus/glenoid fossa fracture. More than 2/3 of shoulder arthroplasties are performed in patients older than 65 years of age (Kim et al., 2011). In fact, shoulder arthroplasty is the third most common joint replacement after knee and hip replacement (Lin et al., 2016). The number of shoulder arthroplasties performed annually in the United States is increasing rapidly and is estimated to number between 55,000 and 80,000 (AAOS, 2016). This reflects the increased wear and tear shoulder arthritis in older patients, the increasing longevity of the population, and the increased success of shoulder joint replacement. It also reflects the growing number of surgical options for shoulder joint replacement or partial replacement.
There are several possible surgical options for complete or partial shoulder joint replacement: (a) anatomic shoulder arthroplasty with complete replacement of the humeral head and glenoid fossa; (b) reverse total shoulder arthroplasty with complete replacement of the shoulder joint; (c) shoulder hemiarthroplasty with replacement of the humeral head; and (d) humeral head resurfacing (Petscavage et al., 2012a, 2012b). Total shoulder arthroplasties are somewhat more common than shoulder hemiarthroplasty or humeral head resurfacing. Kim et al. (2011) estimate in 2008 that approximately 27,000 total shoulder arthroplasties and 20,000 hemiarthroplasties were performed. Reverse total arthroplasty was approved by the United States Food and Drug Administration (FDA) in 2003 and probably is part of the reason why there has been an increased incidence of shoulder arthroplasty in the United States in recent years (Kim et al., 2011).
Modern shoulder arthroplasty started with the pioneering work of Neer in 1955 (Neer, 1955; Walch et al., 2010). With an intact rotator cuff and an unconstrained prosthesis there is a prosthesis survival rate of 97% after 10 years and 84% after 20 years for total shoulder arthroplasty (TSA). For patients who have complex, comminuted proximal humerus fractures (3 or 4 part), how well the greater tuberosity heals determines the outcome of the shoulder arthroplasty (Walch et al., 2010).
The long-term results for other forms of shoulder joint replacement (shoulder hemiarthroplasty, reverse total shoulder arthroplasty, humeral head resurfacing) are somewhat less impressive, but are improving. They are good enough though to provide a variety of shoulder joint surgical options for patients with severe proximal humerus fractures, massive rotator cuff tears, and surgical or bony destruction from tumor or infection, or for patients needing revision of a failed prior shoulder arthroplasty.
Anatomic Shoulder Arthroplasty
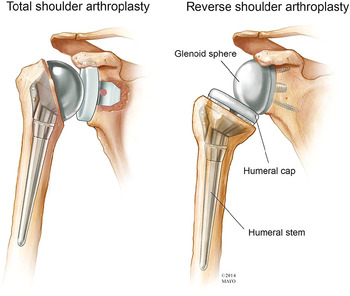
The prosthetic humeral head component is anchored in the proximal humerus in an anatomic shoulder arthroplasty or hemiarthroplasty. Total shoulder arthroplasty (TSA), also known as total shoulder replacement (TSR), requires an intact functioning rotator cuff for the best success. A modular metallic humeral head with a metallic stem articulates with the native glenoid in a shoulder hemiarthroplasty or with a prosthetic glenoid component in a shoulder arthroplasty (Figures 4.1–4.3). The glenoid component may be metal backed and secured by screws or composed of polyethylene and cemented into place. Polyethylene glenoid components have one or more metallic pegs or indwelling wires to make them radiographically visible.
Figure 4.1 Anatomic drawing of total shoulder arthroplasty and reverse total shoulder arthroplasty. Used with permission of Mayo Foundation for Medical Education and Research, all rights reserved.
Figure 4.2 Right shoulder hemiarthroplasty.
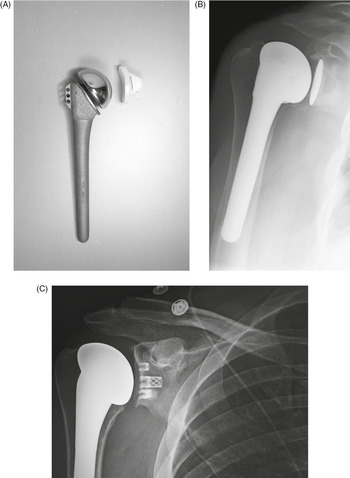
Figure 4.3 (A) Total shoulder prosthesis (arthroplasty). From Benjamin & Lund, 1994. (B) Right total shoulder arthroplasty. The glenoid component is cemented in place, and the humeral component is press-fit. (C) Right total shoulder arthroplasty, different design than those shown in (A) and (B).
The majority of humeral components have successful long-term fixation without bone cement. For the glenoid component, many consider cemented all-polyethelene glenoid components to be the standard for anatomic shoulder arthroplasty (Sanchez-Sotelo, 2011). Two general types of the glenoid components are used: keeled (polyethylene with an indwelling wire loop) and pegged (metal).
The type of total shoulder prosthesis used depends on patient selection, the surgeon’s preference, and the surgeon’s experience (Sanchez-Sotelo, 2011). Shoulder prostheses are much less constrained than hip prostheses. Shoulder prosthesis functionality depends to a great degree on competency of the rotator cuff.
The Total Evolutive Shoulder System (TESS, Biomet) and the Affinis (Mathys) are arthroplasty designs that do not use the traditional long metal stem for the humeral component (Figure 4.4). There is less bone removal and possibly less soft tissue disruption. The humeral head can be positioned despite any deformity of the humeral diaphysis, making these prostheses potentially the most useful in patients with post-traumatic osteoarthritis of the shoulder and deformity in the proximal metaphysis of the humerus (Berth & Pap, 2013). There is also more humeral bone stock should revision surgery become necessary. While the mid-term results are good for this type of design, further investigation is necessary to determine the long-term performance of these prostheses, because the long-term results for the more traditional designs are good.
Figure 4.4 Total Evolutive Shoulder System (TESS) shoulder replacement system.
Foreshortening of the humeral neck commonly occurs following proximal humeral fractures. In all cases, it should be avoided during the placement of a shoulder prosthesis, because it compromises the function of the shoulder (Boileau et al., 2002, 2006; Lazarus et al., 2002). Shoulder arthroplasty is usually a very successful procedure with good pain relief and improvement in shoulder motion and function.
The initial postoperative radiographs frequently show thin radiolucent areas about the prosthesis, particularly about the cemented glenoid component. In most cases they are likely related to difficulty in achieving good cement penetration into the bone and are of no consequence. However, on subsequent radiographs there should be no progression of any radiolucent lines at the implant–bone interfaces, because they may indicate loosening and/or infection. Loosening can occur about either the humeral or glenoid component (Boileau et al., 2002, 2006; Lazarus et al., 2002). At first, it may be difficult to differentiate prosthesis loosening unrelated to infection versus an infection-related surgical complication.
The main complications seen with shoulder arthroplasty are loosening and subluxation or dislocation. Periprosthetic humeral shaft fractures can occur intraoperatively or after surgery secondary to trauma (Boileau et al., 2002, 2006; Lazarus et al., 2002) (Figure 4.5).
Figure 4.5 51-year-old woman with right shoulder prosthesis periprosthetic fracture.
Shoulder Hemiarthroplasty
Shoulder hemiarthroplasty is a less-invasive procedure with somewhat less bony and soft tissue disruption than TSA. The glenoid portion of the shoulder joint is left intact. Shoulder hemiarthroplasty is preferred over a TSA if the glenoid is relatively normal. This is often true with comminuted proximal humerus fractures. It is often the case in humeral head osteonecrosis and in some cases of less-severe osteoarthritis and rheumatoid arthritis. Shoulder hemiarthroplasty is occasionally used as part of the repair for severe rotator cuff tears. Shoulder hemiarthroplasty is also indicated where there is not enough bone stock to support a prosthetic glenoid component and a TSA.
Shoulder hemiarthroplasty is generally not indicated in cases of moderate to severe shoulder osteoarthritis. TSA is superior to shoulder hemiarthroplasty in these cases. TSA gives better pain relief, active range of motion, and patient satisfaction with similar complication rates (Boileau et al., 2006; Radnay et al., 2007). Shoulder hemiarthroplasties performed for osteoarthritis gradually deteriorate from erosion of the native glenoid. There is a significant increased rate of revision surgery with shoulder hemiarthroplasty compared with TSA (Radnay et al., 2007).
The humeral component of a shoulder hemiarthroplasty is often the same as the humeral component of an anatomic total shoulder arthroplasty. It articulates directly with the native glenoid fossa and can be cemented or and press-fit (interference-fit) into place. In cases where there is a rotator cuff tear the prosthetic humeral head is somewhat laterally extended. This helps prevent bony impingement between the greater tuberosity of the humerus and the acromion (Petscavage et al., 2012a).
The hemiarthroplasty design may be used for upper extremity limb-sparing prostheses where the prosthetic humeral head directly articulates with the glenoid fossa, and the humeral limb of the prosthesis is extended replacing all of part of the humerus after widespread surgery for a malignant tumor (Figure 4.6).
Figure 4.6 Limb-sparing prosthesis (hemiarthroplasty). This prosthesis was placed to spare the left upper extremity after widespread surgery for a metastatic bone tumor. The native glenoid is intact and articulates with the prosthetic humeral head.
There are multiple complications associated with shoulder joint hemiarthroplasty, similar to other arthroplasties, including prosthesis dislodgement, infection, and periprosthetic fracture. Specific complications to look for with shoulder hemiarthroplasty include progressive erosion of the glenoid fossa, partial or complete tearing of the rotator cuff with elevation of the prosthetic humeral in respect to the glenoid and acromion, and dislocation of the prosthetic humeral head out of the glenoid fossa (Figure 4.7).
Figure 4.7 Left shoulder hemiarthroplasty anterior dislocation. (A) Axillary view. (B) Frontal view.
Reverse Total Shoulder Arthroplasty
Full-thickness rotator cuff tear is a common chronic ailment in older adults. There is associated superior migration of the humeral head, unopposed deltoid contraction, and ineffectiveness of the glenohumeral fulcrum mechanism. This causes one to lose the ability to raise the arm above the horizontal, so-called shoulder “pseudoparalysis” (Petscavage et al., 2012b; Roberts et al., 2007). There is associated secondary shoulder arthropathy and pain.
The prime indication for reverse total shoulder arthroplasty (RTSA or RSA) is a full-thickness rotator cuff tear. RTSA is sometimes used for acute three- or four-part proximal humerus fractures, shoulder reconstruction after tumor surgery, and shoulder revision arthroplasty (Walch et al., 2010). An intact deltoid muscle is needed for reverse total shoulder arthroplasty (Lin et al., 2016).
The reverse approach to shoulder arthroplasty was introduced by the French surgeon Paul M. Grammont and approved in the United States in 2004 (AAOS, 2016). About half of the arthroplasties now being performed use the reverse design.
RTSA reverses the ball and socket relationship of the shoulder joint, the prosthetic glenoid component being a ball and the humeral component a socket (Roberts et al., 2007; Figure 4.8). Because the joint center is moved distally and medially, there is more control of the shoulder joint motion by the deltoid muscle. This helps overcome the large motion loss from the deficit of the rotator cuff. Active elevation can be restored in patients with an irreparable rotator cuff tear or severe arthritis, but internal and external rotation of the shoulder is limited (Boileau et al., 2006).
Figure 4.8 Reverse total shoulder arthroplasty plus skin staples.
The typical RSA has a cupped humeral component attached to a metal stem. Its radiolucent polyethelene liner sits in the cup and articulates with the metallic head (glenosphere) attaching to a base plate (metaglene) in the glenoid. The base plate is attached to the glenoid by bicortical screws (Roberts et al., 2007). The glenosphere should be aligned fully within the humeral cup, although the separation between the glenosphere and the humeral cup depends on the thickness of the polyethylene liner (Figure 4.9).
Figure 4.9 61-year-old woman with bilateral shoulder prostheses. On the right (A,B) there is a hemiarthroplasty. Notice the suture holes by the neck of the prosthesis for reconstructing the rotator cuff. On the left (C,D) there is a reverse total shoulder arthroplasty. There is possible impingement on the inferior glenoid rim with beginning bone loss, “scapular notching” (arrow).
The most common complication for a RTSA is anterior dislocation which often occurs in the early postoperative period (Petscavage et al., 2012b). In this case, the humerus with its prosthetic cup lies anterior and superior to the glenosphere. This is due to the pull of the deltoid muscle. Posterior dislocation can also occur (Figure 4.10). Either anterior or posterior dislocation may be difficult to appreciate on standard frontal views due to the variable normal distance between the humeral cup and glenosphere depending on the thickness of the polyethylene liner. Any suspected dislocation has to be confirmed on orthogonal views, the axillary view if obtainable being the most reliable.
Figure 4.10 Posterior dislocation of a reverse total shoulder arthroplasty.
Scapular notching is very common after RTSA with bone resorption of the inferior scapular border (Lin et al., 2016; Figure 4.9). This probably results from change in the shoulder’s center of rotation with repetitive contact between the humeral cup of the reverse total shoulder arthroplasty and the inferior scapular border. The importance of the bone resorption is uncertain. In minor cases it is probably best watched, while advanced grades of scapular notching, grades 3 and 4 on the Sirveaux classification scale (see below) may indicate additional surgery (Lin et al., 2016). Scapular spine and acromion fractures are also found with RSA in a small percentage of patients and may be a risk for prosthesis displacement in more severe cases. Radiographic evaluation of a RSA should include searching for scapular notching and subtle scapular spine or acromion fractures.
Sirveaux Scapular Notching Scale (adapted from Sirveaux et al., 2004)
Grade 1 – defect within the inferior pillar of the scapular neck; Grade 2 – erosion of the scapular neck to the level of the inferior fixation screw of the glenosphere; Grade 3 – extension of the erosion to over the lower fixation screw; Grade 4 – extension of erosion to the undersurface of the glenosphere base plate.
Humeral Head Resurfacing
Humeral head resurfacing replaces the humeral joint surface with a metallic covering or metal cap. It preserves the bone of the proximal humerus. The glenoid may be replaced with a polyethylene glenoid replacement, but is often left intact with the humeral metal cup positioned flush against the glenoid surface. Because no humeral osteotomy has been performed, the head–shaft angular relationship of the humerus does not have to be addressed. There is minimum bone resection compared to the other forms of shoulder arthroplasty, minimizing the operative time. There is also a low prevalence of periprosthetic fractures and further surgery for revision arthroplasty is somewhat easier (Burgess et al., 2009). Another advantage to this procedure is fewer metallic-related artifacts on subsequent CT or MRI studies of the shoulder (Petscavage et al., 2012a).
Often a Copland shoulder prosthesis is used for humeral head resurfacing. The Copland prosthesis consists of a metal cup that fits over the resected area of the humeral head. The cup has a metal peg for cementless insertion into the proximal humeral shaft. A similar design uses a threaded screw instead of a peg to insert into the humeral shaft (Figure 4.11). An occasional use for such a prosthesis is to cover a Hill–Sachs or reverse Hill–Sachs deformity (Figure 4.12).
Figure 4.11 Left shoulder resurfacing hemiarthroplasty. Skin staples are also present.
Figure 4.12 Left shoulder head hemi-cap. (A,B) 46-year-old man with left humeral head hemi-cap from treatment of chronic reverse Hill–Sachs deformity. Heterotopic bone is forming about the shoulder joint. (C,D) 48-year-old man with multiple left shoulder dislocations.
The long-term outcome of humeral head resurfacing compared to the other forms of shoulder arthroplasty has yet to be determined. Because humeral head resurfacing produces less bony and soft tissue disruption, it has potential for better long-term results and is a particular option for shoulder replacement in younger patients. Partial humeral head resurfacing may have an especial application for patients with advanced osteonecrosis of the humeral head (Uribe & Botto-van-Bemden, 2009). It may also have application in some patients with rheumatoid arthritis affecting the shoulder joint, but it is not suitable for severely damaged joints, particularly if the humeral head is soft or has insufficient bone (Levy et al., 2004).
The metallic humeral head cup should be flush against the articular surface of the glenoid and not displaced anteriorly or posteriorly on axillary or scapular Y views. There should be minimal lucency about the metallic peg or screw which inserts into the humeral shaft (Petscavage et al., 2012a).
Elbow Arthroplasty
One of the biggest challenges in joint arthroplasty is elbow arthroplasty, because the forces transmitted across the elbow are amplified by the long lever arm of the forearm, and there is limited bone stock about the elbow. The main indications for elbow arthroplasty are similar to those for other joints including advanced osteoarthritis or inflammatory arthritis refractory to medical therapy. Indications also include complex fractures or non-union of the distal humerus (Benjamin & Lund, 1994; Ferlic, 1999; Inglis & Pellicci, 1980). Absolute contraindications for elbow arthroplasty are systemic infection, elbow joint infection, or a neuropathic elbow joint. The goal of elbow joint arthroplasty is to decrease pain and restore a useable range of motion. Modern elbow arthroplasty designs and biomaterials have allowed elbow replacement surgery to become more accepted (Petscavage et al., 2012c).
Arthrodesis of the elbow is a rare procedure that is also part of the surgical armamentarium for chronic elbow pain and disability. It is used for patients with advanced rheumatoid arthritis or patients with failed elbow arthroplasties who do not have adequate bone stock for revision surgery (Berquist, 1995; Ferlic, 1999). Radial head replacement is now more common and is used to treat complex radial head fractures. Capitellar arthroplasty is a newer technique for prevention of osteoarthritis and capitellar erosion in patients with a radial head prosthesis (Petscavage et al., 2012c).
Total Elbow Arthroplasty
Unlike joint arthroplasty in the shoulder, hip, and knee, the main indication for total elbow arthroplasty (TEA) is rheumatoid arthritis rather than degenerative osteoarthritis. In general, prosthetic elbow replacement is avoided in younger patients, because there is an anticipated high rate of failure. The relatively poor bone stock about the elbow joint makes revision arthroplasty in the elbow considerably more difficult. Celli and Morrey (2009) found semiconstrained TEA in young patients was associated with a 22% revision rate. For these reasons, elbow arthroplasty is generally reserved for patients older than 40 (Petscavage et al., 2012c).
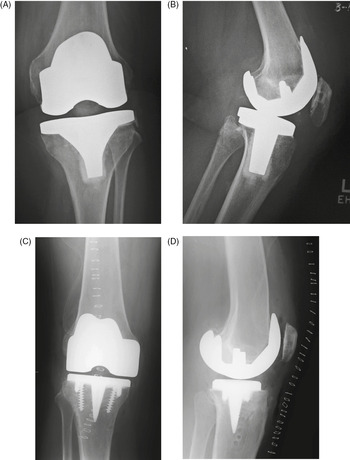
The elbow has motion in flexion and extension as well as pronation and suppination. Three basic types of elbow arthroplasty are: (a) constrained elbow arthroplasty; (b) unconstrained or resurfacing elbow arthroplasty; and (c) semiconstrained elbow arthroplasty (Figures 4.13 and 4.14).
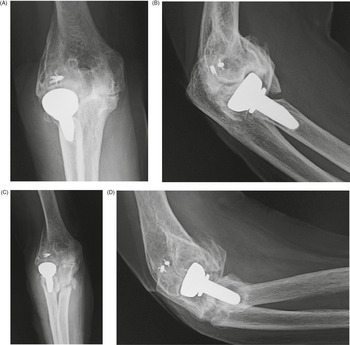
Figure 4.13 (A) Total elbow prostheses. A, constrained stemmed design with hinged articulation. B, resurfacing design with non-constrained (unconstrained) interface (from Benjamin & Lund, 1994). (B) Semiconstrained elbow arthroplasty.
Figure 4.14 Latitude EV total elbow prosthesis (from Tornier). This semiconstrained prosthesis also incorporates a bipolar capitellar–radial head arthroplasty.
A constrained elbow joint replacement consists of a rigid hinge constructed either with metal on metal or metal on high-density polyethyelene. The humeral and ulnar portions of the hinge are connected through a bushing and pin which do not allow varus–valgus motion. The radial head is often resected proximal to the annular ligament. The prosthetic humeral and ulnar stems are cemented into place, and the humeral condyles are resected.
The constrained prosthesis has increased stress at the elbow joint with high rates of osteolysis, loosening, and periprosthetic fractures. The constrained elbow arthroplasty is rarely used these days (Petscavage et al., 2012c).
The semiconstrained elbow (sloppy hinge) prosthesis is the most commonly used total elbow arthroplasty (Figures 4.13–4.16). It is designed to help alleviate some of the loosening problems found with the constrained hinged prosthesis. The humeral and ulnar components are linked together with an axle and bushing arrangement which allows a degree of movement in the varus–valgus (coronal) plane, because there is a circular polyethylene ring which sits between the metal components to decrease friction and allow more movement (Berquist, 1995; Freiberg, 2001;Petscavage et al., 2012c). Some modern elbow arthroplasties also incorporate prosthetic capitellar and radial head components into the design (Figure 4.14).
The humeral components of semiconstrained elbow prostheses are more prone to failure than the ulnar component. In the postoperative period, special attention should be given to anterior migration of the proximal end of the humeral prosthetic stem. Bone remodeling is also common at this location (Benjamin & Lund, 1994).
The unconstrained (unlinked) elbow prosthesis consists of separate humeral and ulnar metal components which articulate through a circular high-density polyethylene component (Figure 4.13). This design relies on the overlying soft tissues, rather than a bushing–axle linkage and pin, to maintain the articulation. The major complication associated with this implant is subluxation or dislocation at the joint itself, although this implant design has the lowest incidence of mechanical loosening from bone.
The elbow is particularly prone to heterotopic bone formation after immobilization or surgery. This complication is not uncommon after elbow prosthesis placement. It may be relatively benign, but it can lead to significant loss of joint mobility and require surgical removal (Figure 4.17).
Figure 4.17 Semiconstrained left elbow arthroplasty with heterotopic bone formation. 35-year-old woman with prior gunshot injury to left upper extremity. Multiple surgeries were performed to remove wooden fragments and shrapnel. Extensive heterotopic bone has developed about the left humerus and left elbow arthroplasty. Portions of previous fixation screws and plates are evident.
Joint prosthesis infection is uncommon but a dreaded complication of elbow arthroplasty (Figure 4.18). Aseptic (non-infectious) prosthetic loosening is more common than prosthesis infection, but the differentiation between prosthesis infection with loosening and aseptic loosening is difficult to determine with imaging alone and may require surgical exploration if there are clinical findings suggesting an infection. While not common, periprosthetic elbow fractures may occur and should be looked for when evaluating radiographic studies of elbow prosthesis placement (Figure 4.19).
Figure 4.18 66-year old man with infected total elbow arthroplasty with eventual placement of a revision total elbow arthroplasty. (A,B) The arrows point to areas of periprosthetic lucency suggesting loosening which was found to result from an infected prosthesis. (C,D) The patient had the prosthesis removed and underwent multiple subsequent surgeries with placement of a revision elbow arthroplasty.
Figure 4.19 Left elbow semiconstrained prosthesis with olecranon periprosthetic fracture.
Elbow Hemiarthroplasty
Elbow hemiarthroplasty is an unusual procedure. There is replacement of one part of the elbow joint with a metallic prosthesis, usually replacement of the distal portion of the humerus (Figure 4.20) (Elbowdoc, 2015). The main indication is severe elbow trauma with a comminuted fracture of the distal portion of the humerus, effectively disrupting the elbow articular surfaces. The procedure is only recommended for elderly individuals or individuals who will be placing minimal demands on the elbow.
Figure 4.20 Left elbow hemiarthroplasty.
Radial Head Replacement (Arthroplasty)
Radial head replacement is most commonly performed for the treatment of comminuted, complex radial head and neck fractures (Berquist, 1995). Radial head fractures represent 33% of all adult elbow fractures. Non-displaced partial fractures (Mason type 1) are treated non-operatively. Mason type 2 fractures representing partial marginal fractures involving a small part of the articular surface or with > 2 mm of displacement are treated with open reduction and internal fixation (Ha et al., 2012). Comminuted fractures of the entire radial head (Mason type 3) and radial head fractures with radius dislocation (Mason type 4) are more difficult to treat.
Internal fixation with a plate and screws or radial head arthroplasty is preferred over radial head excision for those patients with comminuted, unstable fractures (Petscavage et al., 2012c). Radial head arthroplasty if successful in this situation maintains elbow stability and range of motion by preserving the radius length and providing a functioning joint at the prosthetic radial head.
Silicone was used for the initial radial arthroplasties with the prosthetic radial head and neck component often made of Silastic (Figures 4.21 and 4.22). With silicone products there is a high rate of failure with loosening, periprosthetic fracture, and silicone-related synovitis. These types of arthroplasties are no longer being performed.
Figure 4.21 Silicone replacement of radial head and neck.
Currently, radial head implants are composed of titanium, cobalt chromium alloy, or pyrocarbon. Pyrocarbon has an elastic modulus similar to cortical bone helping with bone stress transfer away from the radial head (Petscavage et al., 2012c). Unipolar and bipolar designs are used. The unipolar design has a smooth stem either press-fit or cemented into the proximal radius. The radial head itself centers on the native capitellum.
The bipolar design permits semiconstrained articulation between the prosthetic radial head and the fixed metal stem acting as the radial neck (Figures 4.23, 4.24, and 4.14). The articulation between the metal or pyrocarbon radial head and metallic stem is facilitated by a mobile polyethylene bearing at the head neck junction (Ha et al., 2012; Petscavage et al., 2012c).
Figure 4.23 (A) Bipolar radial head prosthesis, tension band wiring, and two Kirschner wires. (B,C) 57-year-old woman who sustained left radial head and neck fractures eventually treated by a bipolar radial head prosthesis.
Figure 4.24 57-year-old woman with radial head prosthesis and capitellar suture anchor. In 2011 she had a right elbow dislocation with a comminuted radial head fracture. A bipolar radial head prosthesis was placed to treat post-traumatic arthritis. Initial radiographs obtained in 2014 (A,B) showed the prosthesis to be well-seated. Later radiographs (C,D) in 2015 show prominent periprosthetic lucency around the prosthetic stem indicating non-infectious aseptic loosening. There are also chronic fractures of the proximal portions of the right radius and ulna.
The main complications from radial head replacement are heterotopic bone formation about the elbow, secondary radiocapitellar osteoarthritis, and disengagement of the radial head implant (Figure 4.24). Periarticular fracture and infection are less-common complications. The bipolar design may cause less long-term radiocapitellar osteoarthritis compared to the unipolar design. Ha et al. (2012) found that by 9 months after surgery 50% of radial head implants showed radiographic complications. Revision surgery or removal of the implant was required in 24% of the patients they studied.
Sometimes capitellar resurfacing arthroplasty replaces the articular surface of the capitellum. A capitellar resurfacing arthroplasty is composed of cobalt chromium. It consists of a one piece unit with a peg to be press-fit into the distal humerus and a convex, spherical articular surface to articulate with the radial head. Capitellar resurfacing arthroplasty is preventative to reduce the likelihood of capitellar osteoarthritis or erosion in patients with radial head deformation or a radial head arthroplasty (Figure 4.25). Capitellar resurfacing arthroplasty may also be used by itself in patients with localized injury to the capitellar articular cartilage or localized malformation of the capitellum.
Figure 4.25 Radiocapitellar arthroplasty. 74-year-old woman with severe comminuted right radial head fracture treated by removal of the radial head and placement of a radiocapitellar arthroplasty.
Wrist Arthroplasty
Wrist arthrodesis, either partial or total, is the standard surgical treatment for a painful arthritic wrist (Figures 4.26 and 4.27). However, total wrist arthroplasty (TWA) may be applicable in some cases, because improvements in biomaterial technology and surgical technique have made wrist arthroplasty a good alternative for patients who might have had an arthrodesis. Wrist arthroplasty offers increased wrist function, and the more modern prostheses and surgical techniques promise fewer associated complications and a longer functional lifespan for the prosthesis.
Figure 4.26 Wrist arthrodesis with low-contact dynamic compression plate.
Figure 4.27 Spider plate. The scaphoid has been removed. The spider plate transfixes the lunate, triquetrum, capitate, and hamate which are partially fused.
Total wrist arthroplasty is designed to reduce pain and improve function in patients with disabling wrist destruction from rheumatoid arthritis, post-traumatic arthritis, and advanced degenerative osteoarthritis (Nair, 2014). The contraindications for TWA and for arthroplasty in the finger joints are not clearly defined. Acute and chronic wrist and hand infections preclude arthroplasty surgery. It is usually precluded in patients with lack of sensory or motor function and in patients with particularly poor bone stock. Chronic severe volar and ulnar subluxation at the wrist as well as the need for weight bearing through the wrist joint (use of a cane or walker) are also considered contraindications. Flail non-functional digits with swan-neck deformity, failed arthroplasties of the metacarpophalangeal (MCP) or interphalangeal joints, and lupus arthropathy are also relative contraindications for wrist arthroplasty or for further surgery in the hand (Berquist, 1995;Taljanovic et al., 2003).
Intact extensor carpi radialis brevis and longus tendons is requisite for TWA. The carpal height and alignment must be maintained or reconstructed as part of the arthroplasty. The third metacarpal bone is most important. It is often used as a center of rotation in the coronal plane. Stemmed distal prosthetic components are usually inserted into the third metacarpal.
The carpal alignment should be restored to the neutral position in relationship with the radius, but palmar and dorsal flexion is acceptable on the lateral view (Taljanovic et al., 2003). Wrist extension with ulnar deviation for a power grip is probably the most important motion of the wrist to be preserved or restored as much as possible (Taljanovic et al., 2003).
Wrist arthroplasties typically allow 60° of extension, 40° of flexion, and 20° of radial and ulnar deviation (Berger et al., 1999; Berquist, 1995;Carlson & Simons, 1998). The distal ulna is always resected to avoid ulnar abutment that occurs after resecting the distal radius. The proximal radial component of the prosthesis is usually not cemented into the radius, while the distal component, which usually goes into the third metacarpal, is often cemented.
There are several designs for wrist and finger arthroplasties with evolution of the designs over the last several decades (Nair, 2014). This evolution has been stimulated by two factors. First, arthroplasty in the wrist and hand provide good relief from pain and improved function for patients with severe, destructive arthritis. Second, wrist and hand arthroplasties, while initially successful, have poor long-term results with osteolysis, subsidence, and implant fractures after 10 years. This is particularly true of the first-generation implants from the 1960s and 1970s which used a Silastic spacer only (Figures 4.22, 4.28, and 4.29) (Calenoff & Stromberg, 1973;Swanson, 1968).
Figure 4.28 Silastic joint implants. 55-year-old woman with severe rheumatoid arthritis. Silastic implants replace the 2nd through 5th metacarpophalangeal joints. There is an arthrodesis at the thumb metacarpophalangeal joint with three K-wires, a tension band, and a splint.
Figure 4.29 Second metacarpophalangeal joint silicone arthroplasty.
Second-generation wrist implants used metal ball and socket or hemispherical designs, but still had considerable soft tissue and mechanical problems. Third-generation wrist implants have trispherical and other articular surfaces (Figures 4.30 and 4.31). Fourth-generation wrist implants are mainly uncemented and use porous surfaces for better osteointegration (Nair, 2014). It can be difficult to distinguish the various generation of wrist and hand arthroplasties, and their exact names are hard to remember and are unimportant per se. It is important to understand their function and potential complications.
Figure 4.30 Third-generation “biaxial” total wrist prosthesis. 52-year-old woman with rheumatoid arthritis and left total wrist prosthesis (A) which failed three years later and was replaced by a wrist arthrodesis (B). The distal stem of the prosthesis protruded out of the left 3rd metacarpal. The radiograph of the left wrist arthrodesis shows 2nd–5th metacarpophalangeal Swanson silicone arthroplasties, most of which are at least partially displaced.
Figure 4.31 Same patient as Figure 4.30. 52-year-old woman with severe rheumatoid arthritis in the right hand and wrist. There are multiple failed metacarpophalangeal (MCP) Swanson silicone prostheses with partial displacement and bone fracturing. There is surgical arthrodesis at the right index finger MCP joint and bony fusion from long-standing arthritis in the wrist and thumb MCP joint. In her left hand and wrist there was failure of a wrist prosthesis and failure of MCP joint arthroplasties.
The terms elastomer, silicone, and Silastic are commonly used in describing joint implants, especially implants in the hand, wrist, and foot for treatment of severe rheumatoid arthritis. Elastomer is a type of synthetic rubber, and silicone and Silastic are types of polymers. As a practical matter these materials cannot be distinguished from each other with certainty on radiographs. Silastic is used more than elastomer and silicone in current orthopedic practice and can be found in repair of almost any deformity in any joint. While it is probably not technically correct to do so, it is not that uncommon for authors to use silicone and Silastic interchangeably. Another term often encountered is UHMWPE (ultrahigh molecular weight polyethylene). It is often used for articular surface interfaces in many joint implants.
The modern era for wrist and finger arthroplasty began in the 1960s with the first-generation implants. Specifically, in 1968, Swanson announced a double-stemmed, flexible-hinge silicone wrist prosthesis (Swanson, 1968). This first-generation prosthesis was basically a spacer around which scar tissue formed with soft tissue stability to the joint. This prosthetic joint gave good relief of symptoms and range of motion, but as noted above, it had a high complication rate. It and similar early silicone joints are no longer used.
In the 1970s, Muely and Volz simultaneously invented metal and plastic wrist devices for cement fixation of the components. These second-generation designs had metal components for insertion into the second and third metacarpals as well as into the distal radius. Loosening of the distal component occurred in over 50% of these prostheses (Figure 4.32). In 1973, Volz introduced an articulated, non-hinged prosthesis which functioned as a dorsopalmar tracking device. No rotational motion was allowed. While this design had fewer complications, tendon balance and dislocations continued to be significant problems (Berger et al., 1999; Berquist, 1995; Carlson & Simons, 1998).
Figure 4.32 Second-generation total wrist arthroplasty and Silastic metacarpophalangeal (MCP) implants. The wrist is malaligned in ulnar deviation. There is extensive remodeling in the long (3rd) finger metacarpal. Metal grommets with Silastic implants are at the thumb and long finger MCP joints.
To overcome the significant problems with ball-and-socket type wrist arthroplasty, a new third-generation, semiconstrained, transversely oriented, ellipsoid wrist prosthesis was introduced by Beckenbaugh in 1982, and it is known as the biaxial total wrist arthroplasty (Figure 4.30). The prosthetic stems are coated with porous material to enhance cement fixation or eliminate the need for it. This prosthesis has a mean cumulative survival for revision after seven years of 81% with patients having good pain relief and improved motion. However, there is a high complication rate as with earlier total wrist arthroplasty designs and a worrying amount of loosening after 5 years (van Harlingen et al., 2011).
Numerous additional “fourth-generation” designs for total wrist arthroplasty have been proposed in an attempt to solve the complications with previous models. Most fourth-generation implants are uncemented, although many offer the option to use cement for fixation (Weiss et al., 2013). A typical fourth-generation design has three components: radial stem, carpal plate, and carpal ball (Figures 4.33 and 4.34). Another common design uses a silicone elastomere implant at the wrist together with a titanium metal grommet (Figure 4.35).
Figure 4.33 Drawing of ReMotion (Stryker) total wrist arthroplasty, fourth-generation total wrist prosthesis.
Figure 4.34 Typical wrist arthroplasty components.
Figure 4.35 67-year-old woman with advanced, destructive rheumatoid arthritis in the hands and wrists. (A) There are right hand and wrist silicone and titanium metal grommet prostheses. (B) Swanson silicone and metal joint prostheses. There are left radiocarpal and 2nd–5th metacarpophalangeal joint arthroplasties using silicone with titanium grommets.
It is difficult to keep track of these different designs. What is important is to realize that radiocarpal arthroplasty is used in patients with end-stage destructive arthritis, usually rheumatoid arthritis, to good initial effect, but there are long-term complications with osteolysis, loosening, and disruption of the prosthesis. All elbow, wrist, and finger arthroplasties suffer from these possibilities, and imaging evaluation of them should always look for early signs of prosthesis failure.
Occasionally, a limited arthroplasty is performed in the carpus with replacement of one or more of the carpal bones, most often the scaphoid or lunate (Figure 4.36). Sometimes a limited carpal bone fusion is performed using a SLIC (scapholuntate intercarpal) screw fixation system (Figure 4.37). In general, these procedures have poor outcomes because of the difficulty in maintaining the intricate balance of the wrist, which relies on both the bones and the functional complex system of intrinsic and extrinsic wrist ligaments. Silastic and metal arthroplasties of the thumb carpometacarpal joint after the trapezium resection have sometimes been performed. As with other arthroplasties involving the wrist, they have had variable results.
Figure 4.36 Silicone trapezium prosthesis.
Figure 4.37 Flexible wrist carpal bone fusion screw (SLIC screw repair system).
On rare occasions, the distal ulna is resected due to significant trauma or for treatment of a malignant tumor. To restore as much function as possible for the forearm and wrist, there may be reconstruction of the distal radioulnar (DRU) joint with a variety materials – methylmethacrylate, Silastic polymer, or metal (Figures 4.38–4.40). In these situations a hemiarthroplasty is performed with resection of the ulnar head (or even the distal ulnar shaft) and articulation of the prosthetic ulnar head with the native carpal bones. A more complete distal radioulnar joint prosthesis is the APTIS DRUJ prosthesis, which is an enclosed-joint, semiconstrained prosthesis for the DRU joint in which there is end-stage wrist arthritis from trauma or inflammatory change (Figure 4.41). The long-term outcome of these procedures remains to be seen, but they are often the only possibility for the patient to have preservation and meaningful use of the upper extremity.
Figure 4.38 Distal ulna and distal radioulnar joint reconstruction with Rush rod and methyl methacrylate. 66-year-old woman with distal ulnar resection for treatment of a leiomyosarcoma.
Figure 4.39 Silastic ulnar head prosthesis in a patient with end-stage rheumatoid arthritis.
Figure 4.40 Distal radioulnar joint hemiarthroplasty. 66-year-old man who fell from ladder with multiple injuries to the left elbow, left forearm, and left wrist. A distal radioulnar hemiarthroplasty restores the ulnar aspect of the left wrist joint. There are fracture fixation plates in the radial and ulnar shafts. A variable-pitch, headless screw stabilizes a scaphoid fracture.
Figure 4.41 APTIS DRUJ (distal radioulnar joint) prosthesis. 70-year-old woman with past fractures of distal radius and ulna and development of positive ulnar variance and disabling ulnar abutment syndrome.
Hand Arthroplasty
Metacarpophalangeal (MCP) and proximal interphalangeal joint (PIP) arthroplasties are performed for severe joint destruction, most commonly from rheumatoid arthritis. Both silicone and metal prosthetic designs are used (Figure 4.28). These may be stand-alone procedures or used in combination with surgery in other joints, most often joint arthrodesis in the interphalangeal joints (Figure 4.42).
Figure 4.42 Herbert screws used for proximal interphalangeal joint arthrodesis.
An arthroplasty is occasionally performed for an isolated MCP or PIP joint affected by osteoarthritis (Figures 4.29 and 4.43). In cases of rheumatoid arthritis, arthroplasty of the PIP joints is rarely indicated. If surgery is contemplated, arthrodesis is usually performed. In addition, arthrodesis is much more commonly performed than arthroplasty for arthritic pain relief in the distal interphalangeal joints.
Figure 4.43 Left second metacarpophalangeal joint combined metal silicone arthroplasty. 75-year-old man with severe osteoarthritis at the left index finger metacarpophalangeal joint.
There are numerous designs for MCP and PIP joint arthroplasty. Sometimes resection arthroplasty with a silicone elastomere spacer placement is performed in the MCP or PIP joints (Figure 4.29). Surgical fusion of the MCP joints is rarely performed. The modern Swanson design for MCP implants and PIP implants remains standard (Figure 4.35). This is a silicone prosthesis that is often used with circumferential titanium grommets if there is adequate bone stock. Variations of this design are found in other types of MCP joint arthroplasties with or without metal grommets (Figure 4.44).
Figure 4.44 NeuFlex MCP Joint Implant System. Middle-aged woman with rheumatoid arthritis. The implants contain a flexible, tear-resistant silicone rubber.
Arthroplasty for the MCP and PIP joints can provide good pain relief and sometimes restore mobility (Bales et al., 2014). Unfortunately, the long-term outcome has been relatively unchanged for several decades. The pain relief and range ofmotion achieved after surgery is good, but it does not mirror the success of large-joint arthroplasty (Adkinson & Chung, 2014). The long-term hope of many investigators and surgeons is for innovations in biotechnology to make joint prostheses obsolete, but this point is not close at hand, and we will see ever more designs for joint arthroplasties, particularly in the upper extremity where they have not received the widespread usage and success evident with hip and knee replacement.
Forearm, wrist, hand, and finger reconstructions are occasionally performed using artificial tendons. The artificial tendons are sometimes opaque enough to be visible on radiographs or may be impregnated with barium for visibility (Figure 4.45). They are also visible on CT and MRI imaging and may produce strange imaging effects or artifacts that are difficult to initially recognize if one does not have a history of such tendon placement.
Figure 4.45 Flexor digitorum Hunter Rod tendon reconstruction. (A) 64-year-old man with planned staged flexor digitorum tendon reconstruction using a Hunter rod (arrow). (B) Another patient with Hunter rod reconstruction.
Hip Arthroplasty
Modern total hip arthroplasty was developed by the British surgeon John Charnley in the 1960s (Charnley, [1964]2010; Mulcahy & Chew, 2012a). It represented a milestone in the development of modern joint arthroplasty. The first successful joint arthroplasty showing decent long-term results followed the work of Charnley. Disabling pain from severe osteoarthritis is the main indication for total hip arthroplasty (THA). Hip destruction from osteonecrosis, inflammatory arthropathy, and infectious arthritis are also common indications for THA, as are hip fractures in the elderly and extensive benign or malignant tumors around the hip. In fact, for patients older than 65 with intracapsular hip (femoral neck) fractures, treatment with hip arthroplasty is sometimes preferred instead of open reduction and internal fixation of the fracture.
An absolute contraindication for hip arthroplasty is an active pelvic, hip, or thigh infection or a systemic infection. Morbid obesity, neurologic dysfunction, and history of a remote infection are relative contraindications. Given the appropriate conditions, total hip arthroplasty may be considered for patients of any age group after skeletal maturity. Although there is a preference for postponing hip arthroplasty as long as possible in young adults, modern arthroplasty designs permit successful hip replacement in patients under 30 (Adelani et al., 2013; Gililland et al., 2013).
Partial Hip Replacement
There are three basic types of hip arthroplasty. Partial hip replacement or hip hemiarthroplasty replaces the femoral head and neck and leaves intact the native acetabulum. Hip hemiarthroplasty may consist of a single metallic unit, a unipolar hemiarthroplasty or endoprosthesis (Figure 4.46), or it may consist of a bipolar hip hemiarthroplasty in which there is replacement of the femoral head and neck and placement of a prosthetic acetabulum which is press-fit into the native acetabulum. The bipolar hip prosthesis acetabulum is a polyethylene-lined metal cup. It fits into a small femoral head, which is locked to the attached metal femoral stem (Mulcahy & Chew, 2012a). The bipolar hip hemiarthroplasty allows motion between the prosthetic femoral head and the polyethylene lined cup as well as between the cup and the native acetabulum (Figure 4.47).
Figure 4.46 (A) Unipolar hip hemiarthroplasties (endoprosthesis). These are single-piece prostheses press-fit into the native acetabulum and the native femur. From Benjamin & Lund, 1994. (B) Unipolar hip hemiarthroplasty (endoprosthesis) with a cemented femoral component. Note the collar abutting the femoral calcar. (C) Modular non-cemented unipolar hip hemiarthroplasty (endoprosthesis). Note the collar abutting the femoral calcar.
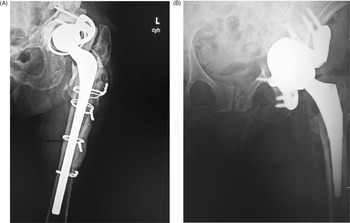
Figure 4.47 (A) Bipolar hemiarthroplasty. A free-riding acetabular cup is press-fit into the native acetabulum. The acetabular cup articulates with a prosthetic femoral head and stem component. From Benjamin & Lund, 1994. (B) Bipolar hemiarthroplasty. From Benjamin & Lund, 1994. (C,D) Bipolar hemiarthroplasty. 92-year-old woman treated for left femoral neck fracture. (E, F) Bipolar hemiarthroplasty with claw plate and cables. 78-year-old woman with prior bipolar hemiarthroplasty presently treated with claw plate and cables for intertrochanteric fracture. (G) Bipolar hip prosthesis with cable plate (cables passed through the plate). The cable plate and wires stabilize a periprosthetic femoral shaft fracture.
The unipolar hemiarthroplasty (endoprosthesis) is typically used in elderly patients with lower life expectancy for treatment of intracapsular hip fractures. There is removal of the femoral head and placement of a press-fit or cemented femoral component with a head diameter that matches that of the acetabulum and articulates directly with the native acetabular articular cartilage. This is a less-traumatic surgery for the patient and enables the patient to have reasonable ambulation quickly after surgery.
The comfort of an endoprosthesis and the hip motion it allows is expected to be less than that with a bipolar hemiarthroplasty or a full total hip arthroplasty, which are more complex surgeries having longer recovery times. However, bipolar hemiarthroplasty and total hip arthroplasty generally give patients better range of motion and are more comfortable.
Because of the motion at both interfaces, bipolar hip hemiarthroplasty tends to be less prone to dislocation than an endoprosthesis. It also theoretically results in less wear of the acetabular articular cartilage that would eventually lead to pain and acetabular protrusio (Benjamin & Lund, 1994; Berquist, 1995). There are variations on the standard bipolar hip prosthesis with claw plates or cerclage wires used to stabilize the stem of the prosthesis (Figure 4.47).
Total Hip Replacement
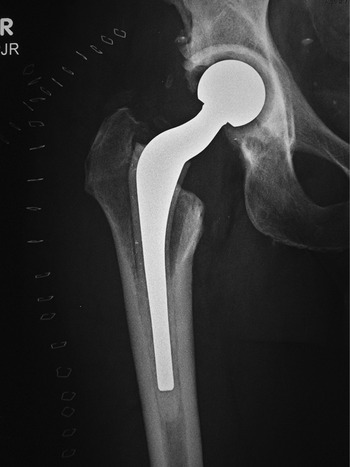
Total hip arthroplasty (THA) is the most common total hip replacement. It uses two components, a stemmed femoral component with a prosthetic femoral head and a prosthetic acetabular component (Figures 4.48 and 4.49). In 2011 there were 306,000 total hip replacements, which is only second to 645,062 total knee replacements (AAOS). There were also estimated to be 105,509 partial hip replacements.
Figure 4.48 (A,B). Modular non-cemented total hip arthroplasty. Implants consist of a proximally porous-coated stem and prosthetic head and a porous-coated metal acetabular component with a polyethylene liner. From Benjamin & Lund, 1994. (C) Total hip prosthesis femoral components. There is a variation in the head diameter. Two components on the right have ceramic heads. From Benjamin & Lund, 1994.
Figure 4.49 Bilateral total hip arthroplasty. 38-year-old man with advanced bilateral osteoarthritis.
The acetabular and femoral total hip arthroplasty components may be cemented in place or non-cemented. Most modern hip arthroplasty systems are modular. The acetabular shell, acetabular liner, femoral head, and femoral stem are separate pieces (Figure 4.48). This allows the surgeon to customize each prosthesis for size and fit. Unfortunately, this can sometimes create problems at the interfaces between the modular components with disengagement and fretting. Classically, the articulating surface between the femoral head and the acetabular lining is metal (femoral head) against polyethylene (acetabular lining).
Bone cement is used to fill voids between the bone and the implant rather than acting as an adhesive. A plug is placed in the medullary space to keep the cement somewhat contained and allows the cement to be injected under pressure (Mulcahy & Chew, 2012a).
If cementless fixation is used, the component is “press-fit” into place, usually into a cavity somewhat smaller than the component itself. It is common to see fixation screws with the acetabular component which are often included for patients with significant acetabular bone loss or because of surgeon preference (Figure 4.50). Rough or porous surfaces on the prosthetic materials facilitate bone ingrowth and are usually the basis for cementless fixation. The surfaces may be treated with bioactive coatings, such as hydroxyapatite, or with sintered beads, fiber mesh, or porous metals (Mulcahy & Chew, 2012a).
Figure 4.50 Elderly woman with bilateral press-fit total hip arthroplasties. There is a dislocation on the right.
Acetabular components usually have the porous coating over the entire surface of the cup, while femoral components are either partially coated proximally or fully coated (Figures 4.51 and 4.52). The choice of cemented or non-cemented designs depends on patient selection and the surgeon’s preference. Generally, cemented acetabular components are recommended for patients who require acetabular bone grafting due to poor bone stock or due to tumor or injury. Patients who have previously received a high dose of radiation often require cemented acetabular components.
Figure 4.52 Total hip arthroplasty with porous-coated stem. The cerclage wires were placed because a femoral shaft fracture (not visible herein) occurred during the prosthesis placement. There is subcutaneous gas from recent placement of the prosthesis.
For patients younger than 65 with a normal life expectancy and adequate bone mass, cementless femoral stems are preferred (Figure 4.49). It is possible that a femoral stem which has become well ingrown will not require revision, even if the acetabular component and the articulating femoral head may need revision in the future. However, as in most cases with orthopedic surgery, the choice of components and the use of cemented or cementless femoral component depends on the surgeon’s preference and experience. With any type of joint replacement, there is concern for its longevity and the need for ultimate revision or complete replacement. Revision surgery is usually not easy and may not have good results. Therefore, joint replacement is avoided as much as possible in younger patients and put off for as long as is reasonable.
Femoral stems are composed of alloys, usually of titanium or cobalt chromium, but sometimes stainless steel is used. If they are monolithic, there is a single component with both a head and stem. If they are modular, they consist of a separate head and a separate stem which have been assembled together. There may be separate necks and collars, with the collar designed to fit against the femoral calcar. Smooth stems which are highly polished are designed for sinking (subsiding) into position in the cement within the first postoperative month (Mulcahy & Chew, 2012a). Stems with roughened surfaces often have a collar and are designed to increase bonding with cement. Often, the stem has a sagittal plane curve to make it fit the anatomic curve of the femur.
The weight-bearing articulating portion of the prosthesis is the articulation between the acetabulum and the femoral head. The femoral head components are composed of either metal or ceramics. The acetabular lining is most often composed of polyethylene which then articulates with either the ceramic or metallic femoral head. This femoral head metal or ceramic articulation with a polyethylene acetabular liner is called hard-on-soft.
Hard-on-hard articulations are metal-on-metal, ceramic-on-ceramic, or metal-on-ceramic; i.e., both the acetabular and the femoral articulating surfaces are composed of a “hard” substance as opposed to a “soft’ substance, polyethylene. The most common articulation is metal-on-polyethylene, hard-on-soft (Mulcahy & Chew, 2012a). Likewise, the most commonly used types of total hip prostheses in North America are hybrids. They have a cementless acetabular and a cemented femoral component. This is a regional preference, and it may not necessarily be true worldwide. If revision is necessary, removal of a non-cemented prosthetic component is technically simpler. It is less traumatic to the patient and often does not entail as much bone loss (Galante, 1998).
Femoral head component size varies from 22 to 32 mm in diameter. Metal-on-metal systems may use larger diameter heads up to 38 mm (Figures 4.53 and 4.54). The acetabular component orientation varies depending on the surgical approach, bone stock, and the preference of the surgeon.
Figure 4.53 67-year-old woman with right metal-on-metal total hip arthroplasty.
Figure 4.54 (A) 29-year-old woman with bilateral hip dysplasia and placement of right metal-on-metal hip arthroplasty for advanced degenerative arthritis from the hip dysplasia. There is postoperative gas evident as well as a surgical drain. (B,C) 55-year-old woman with right hip metal-on-metal arthroplasty.
Radiography is essential for the evaluation of hip arthroplasty. A standard AP view of the pelvis and a cross-table lateral view of the pelvis are usually recommended (Jacobson & Morag, 2015). The preferred position for the acetabular cup is 40–50° of abduction from the horizontal plane and 20° anteversion from the coronal plane (Figure 4.55). The prosthetic femoral head is evaluated for its vertical and horizontal positions relative to the contralateral normal hip, when present, in a number of ways (Jacobson & Morag, 2015; Manaster, 1996; Mulcahy & Chew, 2012a). One method for evaluating the vertical position of the femoral head is to draw a line (A) tangential to the ischial tuberosities, a line (B) tangential to the tops of the greater tuberosities, and a line (C) through the centers of the femoral heads, whether a prosthetic femoral head or a native femoral head. Ideally, these lines should be parallel. To evaluate the horizontal position of the femoral head, the distances between the femoral heads and the tops of the acetabular tear drops on each side are measured. Ideally, these distances should be equal.
Figure 4.55 Hip arthroplasty evaluation. (A) 38-year-old man with bilateral THA for advanced osteoarthritis. The acetabular cup on an AP view of the pelvis should have 30–50° of lateral inclination, which is measured as the angle between the rim of the acetabular cup and a line tangential to the ischial tuberosities. (B) On a true cross-table lateral view of the hip there is normally 5–25° of anteversion, which is measured as the angle between the rim of the acetabular cup and a line drawn perpendicular to the horizontal surface. (C) The distances from a line C between the centers of the femoral heads to a line running tangential to the ischial tuberosities A should be equal on each side, and the distances between the centers of the femoral heads to a line B running tangential to the tops of the greater trochanters should be equal on each side. Lines A, B, C should be parallel. (D) The distances from the centers of the femoral heads on each side to the tops of the acetabular tear drops should be equal.
Anteversion of the acetabular component is evaluated on the true cross-table lateral radiograph of the hip with normal anteversion being 15–20°, although a range of 5–25° is considered normal. On a true lateral radiograph anteversion can only be truly qualitatively estimated. The apparent angulation is affected by pelvic or thigh rotation and the inherent limitations of cross-table lateral hip radiography. If more exacting measurement of anterversion is required, it should be assessed with CT (Manaster, 1996; Mulcahy & Chew, 2012a).
Regardless of the fixation used, the preferred position of the femoral component is with the stem centered in the femoral canal on the anteroposterior view. The center of rotation of the prosthetic femoral head should be at the level of the greater trochanter. The position of the stem can vary somewhat depending on the patient’s pre-existing anatomy (Manaster, 1996). Surgeons performing hip arthroplasty have their own preferred methods for evaluating the arthroplasty results, usually relying on standard radiography coupled with the individual patient’s clinical presentation.
Hip Prosthesis Failure
Non-infectious (aseptic) loosening of prosthetic components is still the most common cause for hip implant failure and subsequent revision surgery. Loosening may be from mechanical stress with failure of the implant binding to the surrounding bone. There may be degradation of the cement–bone interface. Wear on the articular surfaces can produce tiny polyethylene particles or metallic particles which migrate from the joint and lessen bony healing, giving subsequent osteolysis and eventual implant loosening (Figure 4.56). The failure can occur at the prosthesis–bone interface, prosthesis–cement interface, or cement–bone interface. Progressive development of radiolucent areas greater than 1 mm at these interfaces is worrisome for prosthesis loosening (Figure 4.57).
Figure 4.56 (A) Cemented total hip arthroplasty with focal osteolysis about the stem in zones 2, 5, and 7. From Benjamin & Lund, 1994. (B) Femoral osteolysis and loosening of non-cemented total hip arthroplasty. There is radiolucency (arrow) around the entire femoral stem with a sclerotic margin and eccentric positioning of the stem. There is also thinning of the lateral femoral cortex.
Figure 4.57 A resurfacing hip arthroplasty is present. The polyethylene acetabular component is radiolucent. Non-infectious osteolysis (arrow) is present at the tip of the femoral stem.
The acetabulum has traditionally been divided into three zones: zone 1, superolateral; zone 2, central; zone 3, medial aspect of the bone–acetabular interface (Figure 4.58). A radiolucent area greater than 2 mm in any of these three acetabular zones or superior or medial migration of the acetabular cup is indicative of loosening. A change in inclination of the cup is also indicative of loosening (Figure 4.59).
Figure 4.58 (A) Radiographic zones for acetabular and femoral prostheses on AP view. From Benjamin & Lund, 1994. (B) Radiographic zones for femoral prosthesis on lateral view. (C) Radiographic zones for femoral head resurfacing.
Figure 4.59 Osteolysis and particle disease in right hip implant. There is displacement of the right acetabular implant component and osteolysis from granulomatous particle disease. A left metal-upon-metal hip implant is present.
The femur has traditionally been divided into seven zones (1–7) on the anteroposterior (AP) view: zones 1–3 at the lateral side proximal to distal; zone 4 at the tip of the femoral stem; zones 5–7 at the medial side going distal to proximal. On the lateral view the femur is divided into zones 8–10 at the anterior side proximal to distal; zone 11 at the tip of the femoral stem; and zones 12–14 on the posterior side going distal to proximal (Figure 4.55). In any of these zones on any view, a radiolucent area greater than 2 mm is indicative of loosening (Benjamin & Lund, 1994; Berquist, 1995; Freiberg, 2001; Galante, 1998; Manaster, 1996; Figure 4.56).
Periprosthetic bone remodeling often occurs after hip arthroplasty. There are significant stress alterations in the proximal femur and pelvis after prosthesis implantation with areas of stress shielding, an adaptive atrophy in a region with less mechanical stress than previously. Cortical bone requires continued mechanical stress for it to maintain its integrity. Stress shielding can be seen as one or more areas of focal resorption which are most common in the proximal medial femoral cortex and in the superomedial acetabulum (Benjamin & Lund, 1994; Mulcahy & Chew, 2012a). Because of the alterations in bone stress, distal cortical thickening is commonly seen in the femur. The bony remodeling found around cementless acetabular components are most commonly due to resorption of the subchondral plate and relative osteoporosis in zone 2.
Charnley in the 1960s was the first to recognize focal osteolysis about hip prostheses. It was initially believed to be related to the cement used to anchor the prosthesis. Since then, it has become evident that any small particles (metal, cement, or polyethylene) may play a role initiating osteolysis. One in eight total hip replacements needs revision within 10 years, often because of wear-related complications. Bearing surfaces are made of cobalt–chromium, stainless steel, other metal alloys, ceramics, polyethylene, or a combination of these. Friction between the moving surfaces and corrosion of non-moving areas may produce particulate material and can also result in increased local and systemic metal concentrations (Bradberry et al., 2014).
When local particulate matter becomes prominent and threatens or causes prosthesis failure, it is called particle disease or metallosis (Figures 4.59–4.61). See the discussion of adverse reaction to metal debris in Orthopedic medical devices and cross-sectional imaging: protocols and artifacts – MRI.
Figure 4.60 68-year-old man with particle disease from a worn out left hip prosthesis. Bony destruction (arrows) is in the left supra-acetabular region and in the left greater trochanter with a pathologic fracture.
Figure 4.61 Left hip arthroplasty with polyethylene liner wear and associated particle disease with osteolysis.
The prosthetic femoral head should sit in the center of the prosthetic acetabulum with symmetrical spacing between all aspects of the femoral head and the acetabulum. An eccentric position of the femoral head component within the acetabular component usually signifies abnormal polyethylene wear and eventual prosthesis failure (Figure 4.62). Polyethylene wear can produce a granulomatous reaction with bony osteolysis and possible soft tissue mass which might be misinterpreted as a malignancy. A displaced polyethylene liner has a similar appearance to abnormal polyethylene liner wearing; in any case, distinction between the two is not necessary as an eccentric position of the femoral head component signifies prosthesis failure (Figure 4.63).
Figure 4.62 Right hip arthroplasty polyethylene wear. Note the loss of joint space in the superolateral aspect of the arthroplasty.
Figure 4.63 Displaced polyethylene liner in right hip arthroplasty.
Femoral stem fracture is rare, but was seen more commonly in the past when alloys with relatively low fatigue strength (stainless steel) were used for the prosthesis. Prosthesis breakage has become rare with the introduction of high-strength metal alloys, such as cobalt–chromium alloy, titanium alloyed with aluminum and vanadium, or high-strength stainless steel (Galante, 1998).
Metal-on-metal implants have become particularly controversial (Figure 4.54). DePuy voluntarily recalled the ASR XL metal-on-metal implant worldwide in 2010 because of a high revision rate (Ardaugh et al., 2013; FDA Hip Recalls). In the United Kingdom there was an approximate 13% revision rate for this device within 5 years. Two concerns have developed for metal-on-metal implants – somewhat high failure rates and possible systemic cobalt toxicity from high cobalt blood levels (Figure 4.64). It appears that the greatest risk of systemic cobalt toxicity probably results from abnormal wear of a cobalt-containing revision of a previously failed ceramic prosthesis instead of from primary failure of a metal-on-metal prosthesis (Bradberry et al., 2014). There are also data to suggest that repeated measurement of metal ion (cobalt or chromium) levels does not provide useful information for clinical decision making (Reito et al., 2014).
Figure 4.64 68-year-old woman with left hip metal-on-metal prosthesis. Bony erosions (arrows) are evident on the greater and lesser trochanters from probable metallosis with pseudotumor formation.
The revision rate for a given prosthesis depends on a number of factors including prosthesis design, patient selection, and the surgeon’s experience (Dy et al., 2014). Younger age and low hospital volume among other things increase the risk of undergoing early revision arthroplasty. The probability of undergoing early aseptic (non-infectious) revision is about 4% at 5 years. The probability of having a revision due to sepsis at 5 years is less than 1% (Dy et al., 2014). Long-term follow-up in selected patients suggests both metal-on-metal and metal-on-polyethylene arthroplasties are safe based on the mortality of the recipients during the first 20 postoperative years in comparison with the population at large (Visuri et al., 2010).
Hip instability and dislocation is a feared complication of hip replacement. Fortunately, dislocation is a relatively uncommon complication in primary total hip arthroplasty, occurring in approximately 0.4%–0.8% of cases. It is, unfortunately, much more common after hip revision arthroplasty, occurring in up to 16% of cases (Galante, 1998). Hip dislocation occurring within the first 3 months after surgery is presumed to be caused by laxity of the immature pseudocapsule of the joint and laxity of the soft tissues (Mulcahy & Chew, 2012b). In our experience, most prosthetic hip dislocations are in a posterior direction (Figure 4.65). Late dislocation is often the result of trauma. Early dislocation may often be successfully treated with non-operative reduction, while a late dislocation or a traumatically induced dislocation often requires surgical correction.
Figure 4.65 Dislocated left hip arthroplasty. Most hip arthroplasty dislocations are posterior.
Another common complication after orthopedic surgery is heterotopic bone formation. It is particularly prominent about the elbow and hip and most often seen after fracture fixation around these joints. It may be found in any soft tissue after trauma and/or surgery and is often an incidental finding. It should not be mistaken for an aggressive bone forming soft tissue sarcoma, as might be the case with the early stages of heterotopic bone formation (myositis ossificans) in the thigh adductor musculature. Occasionally, heterotopic bone becomes symptomatic, limiting range of motion, and has to be surgically removed (Figure 4.66).
Figure 4.66 Heterotopic bone formations. (A) 55-year-old man with right hip metal-on-metal arthroplasty and heterotopic bone formation above the greater trochanter. (B) The metal-on-metal prosthesis was removed and replaced with a standard hip prosthesis due to continued hip pain and chronic fluid collection (pseudotumor) around the greater trochanter. A later scout CT image shows increased heterotopic bone formation (arrow) near the revision prosthesis. The heterotopic bone was later removed.
Revision surgery is usually more involved and requires considerably more surgical experience than primary total hip replacement. There is increased cost, morbidity, and technical challenge (Dy et al., 2014). A variety of revision arthroplasty devices have been developed. Typically, there is a longer femoral stem, and there may be cerclage wires or cables to help stabilize the prosthesis (Figure 4.67).
Figure 4.67 Revision total hip arthroplasty. The revision prosthesis was placed because of an earlier periprosthetic fracture. There are two cable wires proximally and two cerclage wires distally.
Some acetabula are so severely compromised with bone loss they cannot be managed using ordinary cups, bone substitute augments, or metal cages. In these cases, allograft-prosthetic composites or custom acetabular components may have to be used (Berasi et al., 2015). The triflange design is a common revision hip replacement (Figure 4.68). These are used in cases of failed prior salvage reconstruction with cage or porous metal construct augments, in cases with large bony defects and possible pelvic discontinuity, and in cases where the hip has had multiple previous surgeries with resulting poor bone stock (Berasi et al., 2015).
Figure 4.68 (A) Triflange acetabular component total hip revision arthroplasty with long femoral stem. (B) Triflange acetabular component total hip revision arthroplasty.
Resurfacing Hip Arthroplasty
Resurfacing hip arthroplasty replaces only the articular surfaces (Figure 4.69). This preserves bone stock in an attempt to reduce postoperative complications. The femoral head may have resurfacing alone, or there may be resurfacing of both the femoral head and the acetabulum. In the Birmingham Hip Resurfacing (BHR) system a metallic femoral cap articulates with a metallic acetabular cup. Metal-on-metal hip resurfacing was developed for younger, more active patients as an alternative to total hip replacement, because more bone stock is preserved and future revision, if necessary, would be easier.
Figure 4.69 Femoral head resurfacing prosthesis.
The metal surfaces are highly polished, and their articulation is lubricated by normal synovial fluid. Because most of the resurfacing hip arthroplasty devices use metal-on-metal surfaces, they remain controversial and are not nearly as common as traditional hip replacement (Marshall et al., 2014). Periprosthetic lucency around a resurfacing hip arthroplasty is evaluated similar to evaluation for a total hip arthroplasty. There are three zones around the femoral head peg numbered 1, 2, and 3 from lateral to medial on the AP view (Figure 4.58).
Infection
A dreaded complication for hip replacement surgery is postoperative infection, deep joint infection (JPI), necessitating prosthesis removal. The infection rate may be as high as 1%–2% for primary total hip replacement and even higher for total hip revision (Mulcahy & Chew, 2012b). In Sweden, the cumulative incidence of primary joint infection within 2 years after primary total hip replacement has been reported to be 0.9%, with Staphylococcus aureus and coagulase-negative staphylococci as the most common bacteria isolated (Lindgren et al., 2014).
Diagnosis of deep joint infections is difficult. Prosthesis loosening from infection may simulate aseptic (non-infectious) loosening and prosthesis failure, which is more common. A rapid time course for osteolysis and an aggressive appearance with periosteal reaction favor the diagnosis of infection (Mulcahy & Chew, 2012b). Adjacent soft-tissue fluid collections are also worrisome for an infection, though they are non-specific. The patient’s clinical picture may determine the distinction between an infected versus non-infected prosthesis with fever, redness, localized swelling, and elevated white blood cell (WBC) count favoring infection. See also the discussion for the diagnosis of osteomyelitis and prosthesis infections in Nuclear Medicine Imaging: Medical Devices and Artifacts.
Infected prostheses are generally treated in a two-stage procedure. The infected prosthesis is removed with hip debridement and drainage of infected fluids. The patient is placed on intravenous antibiotics for 6–12 weeks, and a temporary antibiotic-laden cement spacer (ALCS) or antibiotic-laden hip prosthesis is placed (Figure 4.70). A recent development is an acrylic antibiotic-laden temporary prosthesis, the DePuy PROSTALAC system (Figure 4.71). This system includes a thin polyethylene acetabular liner, a metallic femoral head, and an acrylic antibiotic-laden femoral component. Exactech also makes similar antibiotic spacers/prostheses for the shoulder, knee, and hip.
Figure 4.71 DePuy PROSTALAC total hip replacement. 53-year-old man whose previously infected right hip prosthesis was replaced with the PROSTALAC prosthesis having antibiotic-laden components.
After an interval of 6–12 weeks or longer, the intravenous antibiotics are stopped, and the temporary ABLS or antibiotic-laden temporary prosthesis is removed. Then a new prosthesis is implanted.
Knee Arthroplasty
Total Knee Arthroplasty
Total knee arthroplasty (TKA) (also known as total knee replacement, TKR) was introduced in the 1970s. It is mainly performed for advanced osteoarthritis of the knee. It is also sometimes performed for end-stage inflammatory arthritis of the knee. Total knee replacement is by far the most common joint replacement, with hip joint replacement a distant second. Approximately 4 million adults in the United States have a total knee replacement, representing 4.2% of the population 50 years of age or older. The prevalence of knee replacement is higher among females than males (~1.4:1). It is estimated that over one-half of the United States adults diagnosed with knee osteoarthritis will eventually undergo total knee arthroplasty (Weinstein et al., 2013).
Both hip and knee replacement have a long track record of success. There is good patient satisfaction with pain relief and restoration of more normal motion at the affected joint. If there is proper patient selection and good surgical technique, both hip and knee joint replacement in older adults can give good to excellent results in 95% of patients. Younger patients may have slightly better clinical outcomes after TKA. However, the long-term survival of the TKA is lower in younger patients (<55) compared to older patients (McCalden et al., 2013). The survival rate of a hip or knee implant on average is expected to be 95% at 15 years (Insall, 1993).
There are data to suggest TKA for primary osteoarthritis is more successful and longer-lasting than TKA for post-traumatic (post-fracture) osteoarthritis of the knee (Lunebourg et al., 2014). Post-traumatic arthritis of the knee is quite common after- intra or extra-articular fractures of the femur or the tibia. In this case, articular irregularity, misalignment of the lower limb, and joint instability lead to post-traumatic arthritis. In end-stage post-traumatic arthritis when non-operative treatment has failed, knee arthroplasty is an option. There are increased technical difficulties for this type of surgery including old scars, stiffness, possible malalignment, and ligament imbalance (Lunebourg et al., 2014).
The knee has three articular components – medial and lateral tibiofemoral surfaces and the patellofemoral articulation. The most common knee replacement surgery replaces the femoral articular surfaces with a metallic bicondylar component. The tibial articular surfaces are replaced with a metallic tray carrying a polyethylene surface. The patellar articular surface is also replaced with a patellar polyethylene “button” which may be metal-backed (Mulcahy & Chew, 2013). Usually the anterior cruciate ligament is sacrificed, because most surgical approaches are anterior.
The posterior cruciate ligament (PCL) may or may not be removed. There are considerably more than 100 different knee implant designs, and it is not possible to know the exact name for a given implant. One should understand a knee implant’s basic components and functions and recognize any associatedcomplications. Most modern total knee replacement designs use a cobalt–chrome alloy bicondylar femoral component that articulates with a polyethylene tibia weight-bearing surface. The tibial polythethylene surface is attached to a metallic tray usually composed of a titanium alloy.
A PCL-retaining knee allows for preservation of the posterior cruciate ligament and is a relatively unconstrained design (Figure 4.72). For this type of prosthesis to be successful there must be good bone stock, intact surrounding muscles and ligaments, and a posterior cruciate ligament that remains functional (Mulcahy & Chew, 2013).
Figure 4.72 Posterior cruciate-retaining total knee arthroplasty. (A,B) 68-year-old woman treated for severe left knee osteoarthritis. (C,D) Posterior cruciate-retaining total knee prosthesis with cementless femoral and cemented tibial component and patellar resurfacing. There is a postoperative drain and skin staples.
A posterior-stabilized knee or PCL-substituting knee has removal of the posterior cruciate ligament. This design limits posterior tibial translation in flexion. It contains a posterior cam, deeply dished articular surfaces, plus a third condyle or a central polyethylene post in the posterior middle portion of the tibial insert (Figure 4.73). In flexion this polyethylene post engages a transverse metal cam on the femoral component (Mulcahy & Chew, 2013). If the polyethylene post in the tibial insert does not have a metal backing, it may not be visible on knee radiography. Usually one can differentiate cruciate-substituting from cruciate-retaining knee arthroplasty on lateral views. The cruciate-substituting prosthesis often has a larger “box” or thicker femoral component.
Figure 4.73 Posterior cruciate-substituting total knee prosthesis and patellar resurfacing. Note the large distal femoral box. There is a surgical drain in the suprapatellar space.
The selection of a PCL-retaining or -substituting design is at the discretion of the surgeon. There is no evidence that one design is preferable over the other. Patient satisfaction and implant survival are comparable (Lee et al., 2012). Some surgeons use a knee arthroplasty design that preserves both the anterior and the posterior cruciate ligaments, even if the ACL shows evidence of degeneration (Figures 4.74 and 4.75). The experience with retention of both cruciate ligaments seems similar to other knee arthroplasty designs (Sabouret et al., 2013).
Figure 4.74 64-year-old woman with Biomet XPA bicruciate-preserving total knee arthroplasty.
Figure 4.75 46-year-old man with Biomet Vanguard total knee arthroplasty. This is a bicruciate-retaining prosthesis.
Knee implants can also be described as fixed-bearing implants versus rotating platform implants (Figure 4.76). Fixed-bearing implants have a metal component inserted into the tibia with a polyethylene tray locked into place on top of it. Rotating platform implants have a similar metallic portion inserted into the tibia. The polyethylene surface is placed on a circular stem. The circular stem allows a small amount of rotation of the polyethylene tray on the metallic tibial component giving somewhat more range of motion in the knee compared to the fixed-bearing design (Padgett & Windsor, 2015). In both cases there is a fixed metallic femoral component. It may not be possible to determine which design has been used on radiographs, and the choice between these designs is determined by the surgeon’s experience and preference.
Figure 4.76 (A,B) Fixed-bearing total knee prosthesis. (C–E) Rotating platform knee prosthesis. Images (A–D) from Hospital for Special Surgery. Image (E) from DePuy Synthes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree