5 Liver Ablation
Minimally invasive image-guided tumor ablation therapies, using both thermal and chemical technologies, have been applied most extensively in the treatment of primary and secondary liver malignancies (over 100,000 cases to date). This chapter provides a practical and comprehensive guide to performing tumor ablation in liver malignancies. Given that the most experimental and clinical literature to date using tumor ablation for liver tumors has been reported for radiofrequency (RF)-based systems, our discussion in this chapter is based primarily on this literature. As other thermal therapies, such as microwave-based systems, are also gaining increasing attention as alternative technologies, many of the principles and data presented here also apply to thermal tumor ablation using these technologies as well. This chapter includes a review of the current clinical circumstances in which radiofrequency ablation (RFA) is performed and information on patient selection. Patient preparation prior to the procedure, the technique of performing RFA, and postprocedure patient management and complications are also discussed. Finally, strategies to successfully treat more difficult cases are presented.
♦ Review of Pertinent Radiofrequency Principles as Applied to the Liver
A detailed description of the basic principles of thermal ablation has been presented in previous chapters. A review of several key concepts as they specifically and practically apply to thermal ablation of liver tumors is presented here.
Pearls: Treatment Planning
The entire tumor needs to be heated to >50°C.
An additional 0.5- to 1.0-cm “ablative margin” also needs to be included.
The goal of ablation therapies is to induce coagulation necrosis and cell death of the entire target tumor/tissue within a single treatment session using either thermal or chemical agents. This requires adequate heating of every tumor cell within the volume to tumoricidal temperatures, which in clinical practice usually translates to 50° to 54°C for 4 to 6 minutes at the ablation margin.1 An additional important concept is to include within the treatment zone an additional 0.5- to 1.0-cm margin of normal-appearing surrounding liver parenchyma, referred to as the “ablative margin” ( Fig. 5.1 ). This concept is extrapolated predominantly from the surgical literature and supported by more recent RFA-based studies, which demonstrate significant persistent local residual tumor from microscopic invasion at the tumor edge or micrometastases in liver parenchyma immediately adjacent to the target tumor.
Thermal Ablation
To achieve thermal tumor ablation, coagulation necrosis occurs from heating the target zone above known threshold temperatures to induce cell death (this is achieved at approximately temperatures of >50°C for 4 to 6 minutes, or 60° to 100°C nearly instantaneously (i.e., within a few seconds), with temperatures >100°C resulting in tissue vaporization and charring, ultimately hindering further tissue heating for RF-based systems). Several factors determine the overall coagulation necrosis achieved as is represented by this equation:
Energy deposition × local tissue interactions – heat loss = coagulation necrosis

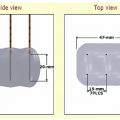
In RFA, tissue heating is achieved by current-induced friction and resistance that occurs immediately around a single or group of electrode applicator(s) within the center of the tumor. The current traverses the body tissue to the grounding pads and circulating back to the RF generator (completing the circuit; Fig. 5.2 ), with the amount of energy being deposited in the target zone determined by the amount of current applied versus the resistance (otherwise known as impedance) to current flow.
Several different commercially available RF devices are commonly used, and they vary based on their power application algorithms and electrode designs. Zones of adequate thermal ablation can be achieved with most commercially available RFA devices when properly used, as described in the instructions for use, though the specific size of the ablation zone and the time required to achieve adequate ablation likely vary between manufacturers and devices. A recent study by Lin et al2 compared four commonly used devices in over 100 patients with primary and secondary hepatic tumors, and found little difference in ablation time and local tumor progression. It is important for operators to be completely familiar with their device of choice, including electrode shapes and deployment techniques, power-input algorithms, and common trouble-shooting issues, so as to permit thorough treatment planning and maximize optimal clinical outcomes. Ultimately, good operator technique and careful patient selection contributes at least as much to treatment success as does the specific electrode choice.
Pearls: Equipment
Know your device!
Cool-Tip RF (Valleylab Inc., Boulder, CO)
Single or cluster needle internally cooled electrodes
Pulsing algorithm and electrode switching
Boston Scientific (Natick, MA)
Expandable noncooled electrodes
Slow increase in current, rising impedance (“roll-off”) end point
RITA/AngioDynamics (Queensbury, NY)
Expandable electrode and saline infusion
Uses temperature end points with stepwise application
Several RF devices commonly used in clinical practice ( Fig. 5.3 ) use different power application algorithms based on current flow, impedance, or time, to deliver RF energy. For example, the Cool-Tip RF electrode system uses a pulsed application that inputs high amounts of current alternating frequently with “off periods” that are triggered by rises in impedance to reduce tissue overheating around the electrode, applied over a 12-minute time period. In contrast, the Boston Scientific device employs two rounds of slowly increasing power to achieve tissue heating, which occurs until tissue impedance starts to rise (thereby limiting further current input) to threshold levels, colloquially termed “roll-off.” Another commonly used system (RITA/AngioDynamics) administers RF energy to set temperature end points (usually 105°C as measured by sensors within the electrode tips) combined with incremental extension of an expandable electrode system. Finally, “switching” technology is an algorithm that allows current application to switch between multiple electrodes during “of periods” of high impedance within the pulsing algorithm, allowing for near-simultaneous heating of tissue around multiple electrode tips. This technology is currently available only with the Cool-Tip® RF system.


Electrode design has also been an important determinant of energy deposition, advances in which have included the use of electrode cooling systems to reduce tissue overheating and charring around the electrode (seen at temperatures of >100°C), and the use of multiple electrodes (either separately placed or using an expandable electrode design). For clinical practice, current electrode designs are paired to specific devices and companies, and RF power algorithms are commonly tailored to specific electrode designs (such that operators cannot interchange electrodes from one device to another, and the greatest efficiencies in use are likely found by following company-recommended application protocols). Several specific electrode designs are available, most commonly divided into those that are needle-like versus multi-tined expandable ( Fig. 5.3 ). The Cool-Tip RF system uses 17-gauge needle-like electrodes as single, cluster, or multiple single electrodes that are internally cooled with ice water (to a temperature of <10°C). The Boston Scientific RF system uses umbrella-shaped expandable electrodes with multiple (10 to 12) tines extending out from the needle shaft. Similarly, the RITA system uses an expandable electrode system paired with cooling saline infusion. Clinicians should be familiar with the shape of the coagulation zone that each system generates. For example, needle-like electrodes induce a more oval ablation zone parallel to the axis of the electrode, whereas expandable electrodes generate ablation zones that are oval-shaped perpendicular to the shaft of the electrode.
The most commonly used commercially available RF systems are monopolar devices, in which the RF current travels from the generator through the electrode(s) into the target tissue, through the body, and then through a grounding pad back to the generator (completing the system circuit). Theoretically, a near-equivalent amount of RF energy returns to the generator through the grounding pad, a thin metallic foil spread over the skin surface (usually the thigh) to dissipate the generated heat and minimize the risk of skin burns. More recently, several bipolar and multipolar devices have also been described, in which two (bipolar) or more (multipolar) electrodes are inserted in the target tumor. RF current is administered through the electrodes, with each in some sense acting as an “active” electrode and a “return” electrode), generating ablative zones both between and around the electrodes, increasing the overall size of the ablation zone. Currently, most of the bipolar devices are commercially available only in Europe and Asia.
Clinicians must have a basic understanding of how various tissue and tumor characteristics interact with tissue heating. In the liver, this interaction is commonly seen in the thermal insulatory effect of cirrhotic liver (over normal liver), which results in improved focal heating of a well-encapsulated hepatocellular carcinoma (HCC; over, for example, a nonencapsulated metastasis in normal liver). Additionally, exophytic tumors surrounded by or adjacent to ascites can be more difficult to completely ablate. Higher amounts of tissue blood flow result in heat loss and have a well-known negative effect on tumor ablation ( Fig. 5.4 ). Within the liver, tumors located centrally or near larger blood vessels (especially with vessels >3 mm in diameter) can be difficult to completely ablate, especially in tissues immediately adjacent to the blood vessels.
Pearls: Tumor Physiology
Improved heating in well-encapsulated HCC
Larger (>3 to 4 mm) adjacent blood vessels reduce heating.
Exophytic tumors surrounded by ascites can be more difficult to heat completely.
Chemical Ablation
Tumor ablation using percutaneous ethanol instillation (PEI) via 20- or 22-gauge needles has long been used to perform ablation for primary hepatic tumors, and remains an attractive option given its low cost and simplicity. Ethanol destroys tissue by two primary mechanisms: (1) as it diffuses into neoplastic cells, alcohol results in immediate dehydration of the cytoplasm, protein denaturation, and consequent coagulation necrosis; and (2) alcohol entering the local circulation leads to necrosis of the vascular endothelium and subsequent platelet aggregation, resulting in vascular thrombosis and ultimately ischemic tissue necrosis. However, success of chemical ablation therapies has been limited by the reported difficulty of achieving uniform diffusion of percutaneously injected drugs over larger tumor volumes due to high intratumoral interstitial pressures that result in poor diffusion of chemical agents throughout the tumor. More recently, given higher reported efficacy and greater uniform tumor coagulation with focal thermal therapies, chemical ablation is used less commonly as an individual agent, but rather usually as an adjuvant agent in tumors difficult to treat with thermal therapies (such as those tumors near critical structures, such as the gallbladder). The treatment of primary hepatocellular carcinoma with ethanol injection has been considerably more successful than the treatment of liver metastases because of tumor characteristics, including softer tissue composition and a capsule or pseudocapsule surrounded by cirrhotic liver compared with the heterogeneous and dense fibrous nature of metastases, which limit diffusion and increase concentration within the target. Efficacy rates of ethanol instillation for hepatic metastases are poor, and therefore not recommended for the treatment of such malignancies.

Pearls: Chemical Ablation
Can be used in HCC; limited efficacy for liver metastases
Limited by poor, heterogeneous diffusion
Mostly now used in cases as an adjuvant to RFA or where RFA cannot be performed
Exophytic tumors surrounded by ascites can be more difficult to heat completely.
♦ Clinical Indications of Ablation
Hepatocellular Carcinoma
Hepatocellular carcinoma is the fifth most common cancer worldwide, with an increasing incidence in the setting of endemic viral hepatitis infections, especially in the Far East. Most cases occur in the setting of underlying liver disease, either in the setting of chronic hepatitis C or alcohol-induced cirrhosis, or in the setting of hepatitis B (where patients have more normal liver function, but are at a higher risk of developing HCC). Traditional and second-generation systemic chemotherapy regimens have been largely unsuccessful in demonstrating a significant survival benefit, though recent antiangiogenic agents, such as Sorafenib, are well tolerated and have promising early results.3 Therefore, the mainstay of curative therapy for HCC is primarily surgical, either liver transplantation or limited hepatic resection. However, only a limited number of patients are candidates for surgical options (on the order of 15 to 30%).4
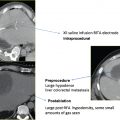
It is within this setting that alternative, minimally invasive and image-guided interventional oncology options, including both intraarterial (transarterial chemoembolization [TACE], and more recently radioembolization) and tumor ablation (RF and other ablation technologies including chemical injection, microwave, and laser) therapies, have gained increasing acceptance as alternative options for focal therapy or as a bridge to liver transplantation. RFA and TACE represent differing but complementary options that can be used separately, in combination (as will be discussed later in this chapter), or sequentially to optimally tailor treatments to a spectrum of individual patient and tumor characteristics. RF tumor ablation is preferred for focal, smaller tumors (<4 to 5 cm in diameter) with fewer foci (no more than three discrete tumors; Fig. 5.5 ). TACE or intraarterial treatments are selected as the primary interventional oncology treatment for patients with more advanced disease that usually entails tumor burdens or features that preclude surgery or tumor ablation (especially infiltrative and multifocal subtypes), or as a temporary therapy in patients awaiting transplantation.

Liver transplantation has emerged as the gold standard for curative treatment of HCC, as it allows for both radical resection of more extensive tumors and treatment of the underlying liver disease (one of the significant contributing factors to higher morbidity and mortality associated with liver resection).4 Given the scarcity of available donors and higher recurrence rates and poor outcomes in patients with more extensive tumor burden, however, several criteria have emerged as mainstays for patient selection. At most centers, patients must fall within the commonly used Milan criteria (single tumor <5 cm or up to three nodules each <3 cm in size) or the less stringent University of California at San Francisco criteria (single tumor <6.5 cm, two to three nodules with the largest <4.5 cm, or a total tumor diameter <8 cm) to qualify for transplant. Patients who develop new focal HCCs while on the waiting list for a donor liver can successfully be treated with RFA as a bridge to transplantation. Several studies have demonstrated reduced dropout rates using this technique, with at least one study demonstrated a 0% dropout rate up to 9 to 10 months compared with a 10 to 30% rate using historical controls.5,6
Pearls: Hepatocellular Carcinoma
Liver transplantation is the gold standard.
Transplant selection:
Milan criteria: single tumor <5 cm, or up to three nodules <3 cm each
UCSF criteria: less commonly used, single tumor <6.5 cm, two or three nodules with largest <4.5 cm, total tumor diameter <8 cm)
Improved heating in well-encapsulated HCC
Larger (>3–4 mm) adjacent blood vessels reduce heating
Exophytic tumors surrounded by ascites can be more difficult to heat completely.
Limited hepatic resection has also been a prominent option in treating focal HCCs. Traditional criteria for liver resection of HCCs, including patients with well-preserved liver function (Childs-Pugh grade A) with small solitary tumors (<4–5 cm), has resulted in 5-year overall survival rates of approximately 37 to 59%.7 Poor baseline liver function (Childs-Pugh grade C) is considered a contraindication to hepatic resection given high perioperative mortality, whereas options for patients with intermediate function (Childs-Pugh grade B) are less clear. However, treating patients who are traditionally considered surgical candidates with percutaneous thermal ablation therapies is gaining increasing acceptance. Advantages of thermal ablation therapies over traditional liver resection for focal HCCs include increased preservation of hepatic function in patients with limited hepatic reserve, reduced morbidity and mortality compared with surgery, and the ability to repeatedly treat new tumor foci that inevitably appear in the setting of underlying cirrhosis.
Results
A substantial number of clinical series have been reported using RFA for the treatment of primary hepatic malignancies, and especially for small hepatomas (<3 cm). In an early study, Livraghi et al8 performed RFA in 114 patients with 126 HCC tumors (measuring >3.1 cm, mean diameter 4.5 cm) and underlying cirrhosis or chronic hepatitis. Complete necrosis was achieved in 47.6% of tumors, near-complete (90 to 99%) necrosis in 31.7%, and partial (50 to 90%) necrosis in the remaining 20.6%. However, treatment of these larger tumors (>4–5 cm) often required multiple sessions of therapy. These earlier studies treating HCC with RFA had mixed outcomes largely due to limitations in patient selection (nonsurgical or unresectable patients were often treated). More recent studies using refined patient selection (smaller HCCs measuring <3 cm, in patients with good underlying liver function, i.e., Childs-Pugh grade A) have reported excellent results, with 5-year survival rates similar to those for hepatic resection. For example, Lencioni et al7 treated 187 patients with Childs-Pugh grade A or B liver disease, with tumor burden falling within the Milan criteria (single focal tumor <5 cm, up to three separate focal tumors <3 cm each), achieving 1-, 3-, and 5-year overall survival rates of 100%, 89%, and 61%, respectively ( Fig. 5.6 ). Interestingly, they also identified a new, separate tumor emergence rate of 81%, underscoring the need for strong liver parenchymal preservation strategies (beyond the fact that the liver was cirrhotic and hence damaged) as repeat treatment of new tumors will likely be required ( Fig. 5.7 ). Similarly, Livraghi et al9 treated 218 patients with early small focal HCCs (<2 cm), using standard outcome variables applicable to surgical series: sustained, local, complete response and complication rates. This study reported a sustained complete local response in 97%, low complication rates (mortality 0%, major complications 1.8%), and an overall 5-year survival of 68%, quite comparable to surgical outcomes.
Comparison of Radiofrequency Ablation to Percutaneous Ethanol Instillation for Hepatocellular Carcinoma
Historically, PEI has been used successfully as a chemical ablative technique for small focal HCCs. More recently, several studies have demonstrated improved treatment with RFA compared with PEI. Livraghi et al10 compared RFA and PEI for the treatment of small HCC tumors in a 86 patients with 112 tumors (mean diameter <3 cm). The authors reported complete necrosis in 47 (90%) of RFA-treated tumors compared with 48 (80%) of PEI-treated tumors, while requiring almost four times fewer treatment sessions, demonstrating equal if not improved results for RFA over ethanol instillation for small hepatomas. More recently, several studies have demonstrated significantly higher 2-year local tumor recurrence rates for focal tumors less than 4 or 5 cm in diameter treated with PEI (33 to 45%), compared with RFA (4 to 18%). Currently, PEI plays a more secondary role, used for tumors (or residual portions of tumors after RFA) that are in difficult locations where there are higher risks of thermal injury, such as near the colon and gallbladder.


♦ Hepatic Metastases
The liver is a very common site of metastatic involvement from distant primary tumors, most often from gastrointestinal tract and breast tumors. “Liver-only” disease is a common concept describing the presence of metastases confined to the liver, without any other extrahepatic foci. These patients in particular are often candidates for attempted curative therapy (either surgery or ablation), and a clear understanding of the existing outcomes literature is a necessary requisite to good patient selection.
Colorectal Metastases
Approximately 30% of patients with colorectal cancer develop synchronous or metachronous hepatic metastases. Conventional chemotherapy has provided only limited relief in patients with metastatic disease, though recent results with newer agents like oxaliplatin and irinotecan, and antiangiogenic agents such as the anti-vascular endothelial growth factor (VEGF) antibody Bevacizumab, have been promising in prolonging patient survival in palliative regimens, and downstaging initially unresectable into resectable tumors. As such, for patients with liver-only disease, surgical resection remains the standard treatment for colorectal metastases, especially given advances in staged local resection combined with liver regenerating strategies such as portal vein ligation or embolization. The optimal 5-year survival after hepatic metastatic resection in well-selected patients is as high as 40 to 50%, with poor prognosis associated with hepatic metastases numbering more than three, extrahepatic disease, tumor diameter >5 cm, and a positive resection margin. When tumor is left behind after surgery, overall survival is similar to that in the unresected group. Generally, extra-hepatic disease is considered a contraindication to surgical intervention, with the exception being patients with liver and lung-only metastases, in whom some studies have demonstrated 5-year survival rates upward of 30% when treated with localized resection in both organs. The largest experience using thermal ablation therapies for hepatic metastases has been in this specific population: patients with colorectal liver-only disease who either do not meet criteria for surgical resection but would still benefit from liver-directed therapy, or in whom RFA is performed as adjunct to staged hepatectomy.
Initial studies using RFA for hepatic colorectal metastases had relatively poor 5-year survival rates (approximately 15%) compared with surgical resection. Subsequent studies have had improved 5-year survival outcomes more closely approximating those of surgical resection, in part due to improvements in patient selection, more accurate and improved follow-up, advances in chemotherapy regimens, and immediate re-treatment of persistent or untreated tumor. A review of the existing literature on RFA for hepatic colorectal metastases remains mixed, as many studies comparing percutaneous therapy to surgical resection contain inherent selection biases (RFA is often performed in nonsurgical patients). Nevertheless, Solbiati et al11 reported early optimistic results in 117 patients with 179 colorectal metastases treated with RFA. Estimated 1-, 2-, and 3-year survival rates were 93%, 69%, and 46% respectively, and were not influenced by the overall number of metastases. Additionally, more recently Gillams and Lees12 reported the long-term outcomes of their experience treating patients with colorectal liver metastases. In their subset of well-selected patients (tumors <5 cm in diameter, no more than five separate tumors, no extrahepatic disease), 5-year survival was between 24 and 33%, approaching that of surgical resection.
In studies where RFA is applied to patients who are otherwise eligible for surgery, results are more promising. Livraghi et al,13 using a “test of time” approach, treated solitary hepatic metastases <4 cm in diameter with RFA initially, rather than with surgical resection. They found a significant portion of their patients developed new intra- and extrahepatic disease, and as such, 50% were spared noncurative surgery, whereas 26% of patients were spared surgery due to curative RFA. Of note, in following patients after RFA, no patients lost eligibility for hepatic resection. More recently, in the study by Gillams and Lees12 in 309 patients treated with RFA for hepatic colorectal metastases, patients with less than five metastases and tumors <5 cm in diameter with no extra-hepatic disease had 3- and 5-year survival rates of 63 and 34%, respectively. In a smaller series of 40 patients by the same group, when treating smaller tumors (mean diameter 2.3 cm), outcomes were even better and closer to reported surgical rates (1-, 3-, and 5-year survival rates of 97%, 84%, and 40%, respectively).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree