5 Perinatal Imaging
5.1 Introduction
Perinatal imaging includes both fetal and neonatal imaging for both congenital defects and acquired abnormalities. Perinatal imaging utilizes ultrasonography to a greater extent than do other applications of neuroradiology, taking advantage of the thin skull and sonographic windows (in particular the anterior fontanelle) of fetuses and neonates, the absence of ionizing radiation in ultrasonography, and the ability to perform the study without sedation and at the patient’s bedside if needed. Because clinical examinations in this age group can be challenging, the appropriate interpretation of imaging studies can significantly aid in the care of these young patients.
5.2 Fetal Imaging
Fetal evaluation of abnormalities of the central nervous system (CNS) is most commonly done to evaluate areas of uncertainty on screening examinations done with ultrasonography at various stages of pregnancy. After an abnormality is identified or suspected on a screening examination, a more detailed fetal sonographic evaluation can be performed to characterize the abnormality. When there is need for further clarification of a suspected finding and/or associated abnormalities, fetal magnetic resonance imaging (MRI) can be performed. Fetal MRI is typically performed at 1.5 teslas or a lower field strength. Recent investigations have begun to use a field strength of 3 teslas for fetal MRI, but the safety and benefits of this have not yet been established.
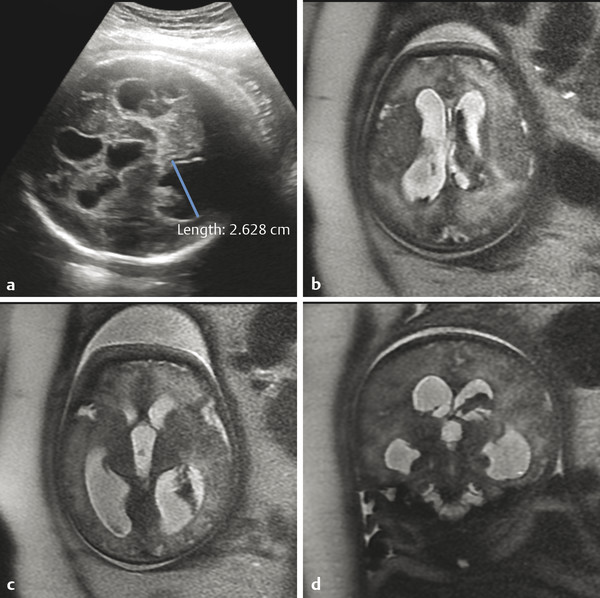
One of the most common indications for more detailed fetal ultrasonography is for ventriculomegaly, which can be associated with a variety of congenital and acquired abnormalities. Fetal ventriculomegaly is typically defined as a transverse dimension of the atrium of the lateral ventricle that exceeds 10 mm. A finding of ventriculomegaly should prompt a detailed survey of the entire CNS 1 ; however, it is occasionally an isolated finding. When ventriculomegaly is present, a follow-up examination should be done to evaluate for its progression, which may result in the need for postnatal shunting (see Chapter 11).
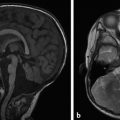
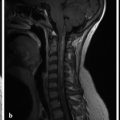
Hydrocephalus can be seen in the setting of germinal matrix hemorrhage (GMH) in utero, which is typically the result of severe maternal stressors, such as an automobile accident or exposure to cocaine (Fig. 5.1). Ventriculomegaly can be related to malformations of the posterior fossa, including a Chiari type II malformation (Fig. 5.2) or a malformation within the Dandy–Walker spectrum (Fig. 5.3), or to supratentorial abnormalities, including agenesis of the corpus callosum (Fig. 5.4). Congenital malformations of the CNS are further discussed in Chapters 3 and 4.
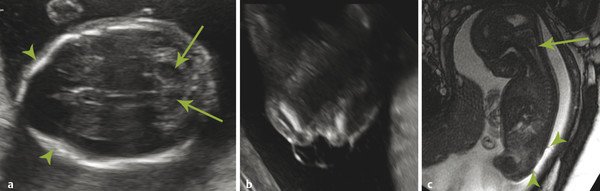
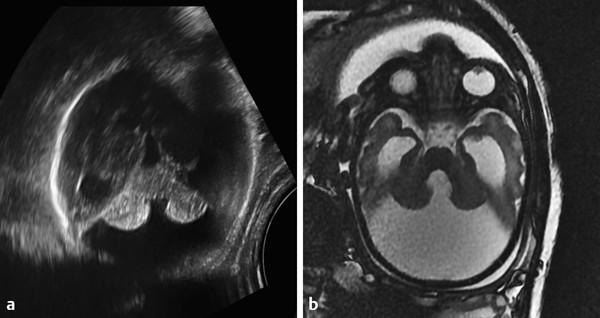
A Chiari type II malformation is nearly always associated with a lumbosacral myelomeningocele (Fig. 5.2), although this can be difficult to see if the back of the fetus is abutting the placenta or the amniotic sac/uterus. A Chiari type II malformation is associated with effacement of the cisterna magna on an axial view of the posterior cranial fossa, resulting in a finding referred to as a “banana sign.” Owing to the low intracranial pressures in fetuses with a Chiari type II malformation, there is slight inward bowing of the frontolateral aspect of the calvarium, resulting in a finding referred to as a “lemon sign.” Cerebellar ectopia through an enlarged foramen magnum cannot always be seen with ultrasonography; it may be better revealed by magnetic resonance imaging (MRI).
Although a Chiari type malformation is marked by a small posterior fossa, the Dandy–Walker spectrum of malformations results in cystic enlargement of the posterior cranial fossa. The extent of vermian hypoplasia in such a malformation can be difficult to determine with ultrasonography, and MRI performs better for this purpose.
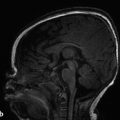
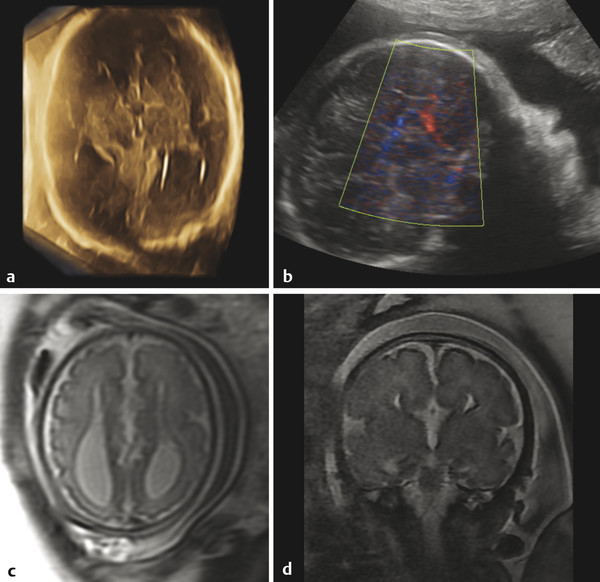
Ventriculomegaly can also be seen with congenital supratentorial malformations, and particularly with agenesis of the corpus callosum (ACC), in which there is preferential enlargement of the atria and occipital horns of the lateral ventricles secondary to a loss of parieto-occipital white matter volume (Fig. 5.4), a condition known as colpocephaly. In ACC, a midsagittal view can show absence of the corpus callosum and a radiating gyral pattern, and a coronal view can show a typical high-riding third ventricle. Agenesis of the corpus callosum is commonly associated with an interhemispheric cyst (“cystic meningeal dysplasia”). 2 When accompanied by a cyst in a female fetus, ACC raises the possibility of Aicardi syndrome, in which there are also abnormalities of the eye.s. Literatur
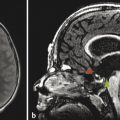
An additional indication for more detailed fetal CNS imaging arises from failure to visualize the septi pellucidi in a screening examination. During development there is typically a cavum septum pellucidum. Absence of the septi pellucidi can be seen in the constellation of findings known as septo-optic dysplasia (SOD), in which the optic nerves are hypoplastic. Both endocrine abnormalities and pituitary malformation, in particular ectopic neurohypophysis, and possibly a schizencephalic cleft, may occur in SOD. Hypoplasia of the optic nerve and an ectopic neurohypophysis can be difficult to confirm on prenatal imaging, and postnatal ophthalmic examination and endocrine testing may be indicated for their detection, as may also be postnatal MRI. At times, absence of the septum pellucidum can be an isolated finding without presumable pathologic consequences, but this must be considered a diagnosis of exclusion.
Septo-optic dysplasia is considered to possibly represent the mildest form of a disorder within the holoprosencephaly spectrum. Although the absence of a septum pellucidum can occasionally be an isolated finding, it should prompt an investigation for other features of disorders within this spectrum of holoprosencephalic disorders
The identification of a more profound parenchymal abnormality makes it important to fully characterize the other (extracranial) findings in the affected fetus, which have prognostic implications for the fetus and possibly genetic implications for any future children. The more severe disorders in the holoprosencephaly spectrum can have an appearance that is confusing to one who is not familiar with the abnormality (refer to Fig. 3.13, Fig. 3.14, Fig. 3.15, and Fig. 3.16). 4 The more severe disorders within the spectrum (e.g. , alobar holoprosencephaly) tend to have poor postnatal prognoses.
A defect in closure of the rostral neural tube can result in an open cranial vault and exposure of the developing tissue of the patient’s central nervous system (CNS) to amniotic fluid, resulting in injury to the tissue and failure of formation of the brain (Fig. 5.5). This condition is known as anencephaly (literally “no brain”).
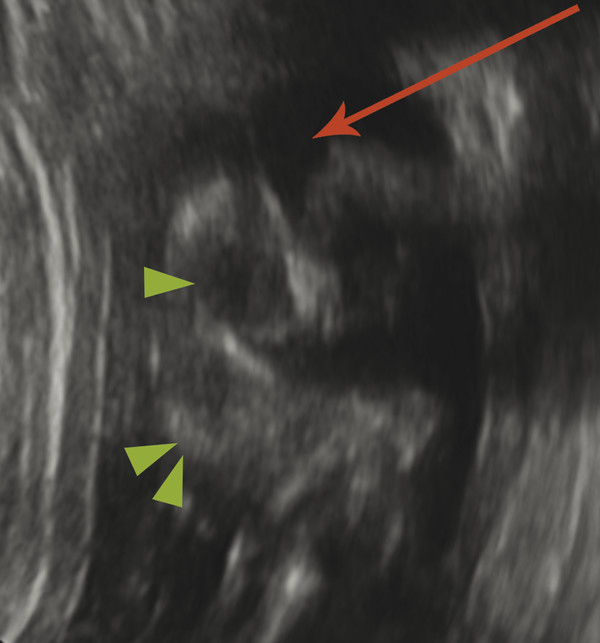
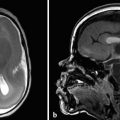
In another condition, known as hydranencephaly (literally “water in place of the brain”) there is in utero occlusion of both internal carotid arteries, resulting in necrosis of nearly the entirety of the supratentorial parenchyma, other than the thalami and possibly the inferior occipital lobes, which may be supplied by the posterior circulation. The brainstem and cerebellum are typically normal. Because the cerebral hemispheres will have originally formed and cleaved, there will be a normal falx cerebri (Fig. 5.6); however, the post natal prognosis is poor. The presence of the falx cerebri can differentiate hydranencephaly from alobar holoprosencephaly.
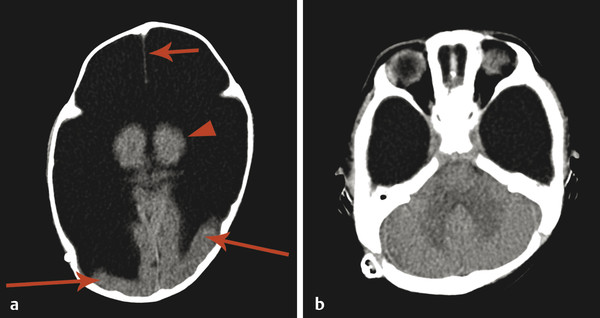
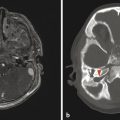
A defect in the calvarium can result in protrusion of the meninges and CSF (meningocele) or brain parenchyma (encephalocele) (Fig. 5.7). An encephalocele can be associated with a phenotype resembling a Chiari type II malformation, known as a Chiari type III malformation. An occipital encephalocele in the setting of renal anomalies and polydactyly can be seen in Meckel–Gruber syndrome.
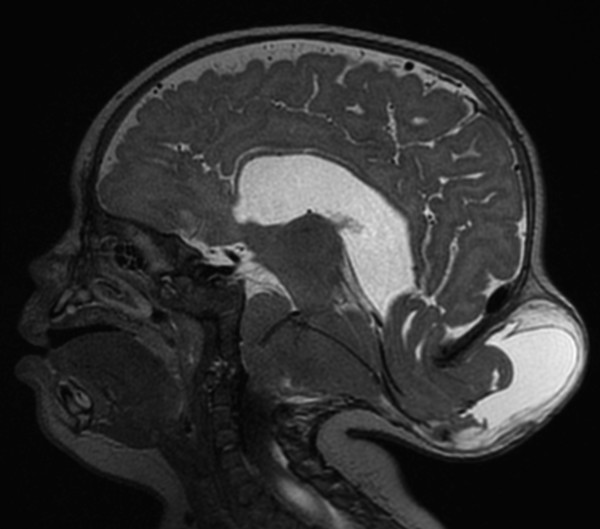
A large central CSF space without a finding of significant parenchyma can be seen in the setting of severe hydrocephalus, which is important to differentiate from other causes because it has the potential for nearly complete normalization after shunting. The thin rim of peripheralized parenchyma may be difficult to see on ultrasound, which is why fetal MRI can help differentiate devastating conditions like alobar holoprosencephaly and hydranencephaly from a potentially treatable severe hydrocephaly.
It is important to note that the normal sulcation pattern of the brain occurs predominantly in the second half of gestation, and it can be very easy to incorrectly diagnose lissencephaly prior to term gestation. This is important to remember for prenatal imaging, as well as for postnatal imaging in very premature infants.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree