5 SPECT and SPECT/CT for the Cardiovascular System
5.1 Introduction
Cardiac single-photon emission computed tomography (SPECT) is widely used for the evaluation of myocardial ischemia, infarction, viability, and function. Scintigraphic images identify selective uptake of a radiotracer by functioning myocardial tissue. Thallium-201 chloride (201Tl) was the original myocardial perfusion tracer used initially with planar imaging and later in dual-isotope protocols with SPECT. Despite excellent first-pass myocardial extraction characteristics, 201Tl provided limited image quality, due to the low-energy photons associated with this radioisotope; in addition, with a half-life of 73 hours, it is associated with the risk of excessive radiation exposure to the patient. 1 Technetium-99 m (99mTc)-sestamibi and 99mTc- tetrofosmin are the most commonly used radiotracers today because of the relatively short halflife of 99mTc (6 hours), easy availability, and high-quality myocardial perfusion and gated images. 2 In this chapter, we will provide a brief overview of 99mTc SPECT myocardial perfusion imaging (MPI) followed by a problem-oriented approach to MPI using SPECT, SPECT/computed tomography (CT), novel semiconductor detector scanner platforms, and software, emphasizing their potential advantages and pitfalls. 3 , 4 , 5 , 6
5.2 Stress Testing and Myocardial Perfusion Imaging
Flow-dependent uptake of the radioactive tracer reveals relative blood flow to the different regions of the myocardium. As myocardial blood flow at rest may remain normal, even with high-grade coronary stenosis, 7 a stress test prior to injection of the radiotracer is needed to produce flow heterogeneity between the normal and the diseased myocardial segments and to noninvasively identify hemodynamically significant coronary artery disease (CAD). Radionuclide MPI is therefore typically performed at rest and during maximal hyperemic stress.
5.3 Stress Testing Protocols
Myocardial stress is achieved either by exercise (preferred) or pharmacologically with regadenoson, adenosine, or dipyridamole (as an alternate to exercise) using standard protocols. 8 Treadmill exercise with the standard Bruce protocol (3-minute stages) is most widely used in the United States, while bicycle exercise is more common in the European Union. Symptom-limited exercise testing, rather than exercise termination due to attainment of age-predicted maximal heart rate (APMHR; 85% of 220 – age), is critical. At least 1-mm horizontal or downsloping ST depression or 1-mm ST elevation (in non-Q-wave leads) in a single lead for three consecutive beats is considered an ischemic response. Exercise-induced ischemic changes typically resolve with termination of exercise. If not, sublingual nitroglycerin 0.4 mg may be administered after ascertaining that there are no contraindications (systolic blood pressure < 90 mm Hg or use of phosphodiesterase inhibitors). If symptoms persist and blood pressure remains stable, then intravenous beta blockers (Lopressor, 5mg, slow intravenous push) can be used to decrease the heart rate and reduce ischemia.
Regadenoson is administered as a nonweight-based slow bolus injection of 0.4mg over 10 seconds, whereas adenosine (140 μg/kg/min to a maximum of 60 mg) and dipyridamole (142 μg/kg/ min to a maximum of 60 mg) are weight-based injections administered over 4 minutes. Adenosine is short acting and typically the effects wear off when infusion is terminated. If wheezing occurs with any of the agents or in patients who are symptomatic or demonstrate ischemic changes, the effects of regadenoson or dipyridamole can be reversed with intravenous aminophylline, 1.0 to 1.5 mg/kg, slow IV push over 2 minutes. 8 Dobutamine is occasionally required when there is a contraindication to the previously mentioned vasodilators, including active wheezing, high-grade atrioventricular block without a pacemaker, or hypotension (systolic blood pressure <90 mm Hg). 8 Intravenous beta blockers as described previously can be used to alleviate tachycardia or adverse effects from dobutamine infusion.
5.4 Myocardial Perfusion Imaging Protocols
Typically, both rest imaging and stress imaging are performed on the same day, with the stress portion of the test performed after the rest imaging has been completed, although stress-first imaging is gaining popularity. 9 A 2-day study is preferred in obese patients (typically men > 250 lb, women >225 lb). 9 For single-day protocols, the radiotracer dose is administered three times for the second scan. If the stress perfusion is normal, the rest scan can be avoided with cost, radiotracer, radiation, and time savings, with excellent outcomes for a normal stress-only MPI. 10 , 11
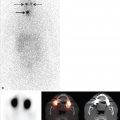
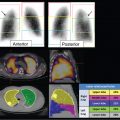
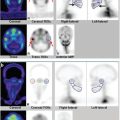
In the figures of this chapter, stress and rest myocardial perfusion images are displayed in alternate rows. The short-axis images (SA, top two rows) are displayed from apex to base (left to right), the horizontal long-axis imaging (HLA, middle two rows) are displayed from the inferior to the anterior wall (left to right), and the vertical long-axis imaging (VLA, bottom two rows) are displayed from septum to the lateral wall (left to right). The polar plots show raw polar plots (left column), polar plots compared to a normal limits database (middle column), and percent peak activity (right column). The top row is stress, the middle row is rest, and the bottom row the difference polar plot. All SPECT scans in this chapter are conventional Anger SPECT (A-SPECT) images, unless otherwise specified. All the cadmium zinc telluride (CZT) SPECT images were acquired using a D-SPECT scanner (Spectrum Dynamics).
Myocardial perfusion may be normal with relative homogeneous perfusion throughout the left ventricle (LV) or abnormal with fixed or reversible perfusion defects. ► Fig. 5.1 demonstrates an example of a reversible defect in the left circumflex artery territory indicating myocardial ischemia. Fixed defects can be associated with normal wall motion (suggesting artifact) or abnormal wall motion (suggesting scar or hibernating myocardium). Identifying and troubleshooting potential artifacts on cardiac SPECT are necessary for accurate image interpretation.
SPECT MPI is highly accurate (83%; sensitivity: 85%; specificity: 72%) and is comparable with (or better than) other noninvasive tests for the evaluation of ischemic heart disease. 12 Indeed, the major advantage of SPECT MPI is that perfusion defect size, severity, location, and reversibility not only guide coronary revascularization (► Fig. 5.1) but also provide powerful prognostic information about risk of future cardiac death or myocardial infarction (MI). 12 However, the high diagnostic and prognostic value of SPECT MPI relies on accurate scan interpretation; false-positive rate remains problematic, particularly in women and in obese patients, and the optimal specificity of MPI depends on accurate identification of artifacts. 13 The advent of 99mTc radiotracers and electrocardiographic (ECG) gated SPECT MPI in the late 1990s allowed for accurate measurements of ventricular wall motion and thickening, ejection fraction, and ventricular volumes, and was a major step forward in distinguishing real defects from attenuation artifact. 14 , 15 Repeat image acquisition and motion correction software (for minor simple motion) help with motion artifacts. 9

Also, after several decades of using the robust conventional A-SPECT scanners, with limited count sensitivity from the collimators, there have been several major recent advances in SPECT technology. High-sensitivity semiconductor detectors with cardiofocal collimation (D-SPECT, Spectrum Dynamics [all CZT SPECT figures in this chapter]; Alcyone, GE; or Cardius, Digirad), attenuation-corrected SPECT (radionuclide or CT), and novel software advances (fully iterative reconstruction with resolution recovery and scatter correction) have improved quality of images obtained with low and very low radiation dose SPECT MPI. 34 , 5 , 6 As with conventional SPECT technology, this new technology poses specific imaging challenges that will be discussed in this chapter.
5.5 Troubleshooting Traditional and Novel SPECT MPI
Rest and stress MPI should be interpreted systematically, starting with a review of the rotating projection images and followed by a review of the static perfusion images, polar plots, and gated images. A perfusion defect seen at rest and at stress is called a fixed defect, whereas a perfusion defect seen at stress but not at rest is called a reversible perfusion defect. The differential diagnosis of fixed and reversible perfusion defects is listed in ► Table 5.1.
Perfusion pattern | Differential diagnosis |
Fixed defect | Attenuation Scar Hibernation |
Reversible defect | Motion Misregistration Scanner failure Ischemia |
5.6 Fixed Anterior Wall Abnormalities
The differential diagnosis of fixed defects in the anterior wall includes breast attenuation artifact in female or obese male patients, or infarction or hibernating myocardium in the territory of the left anterior descending (LAD) artery. It is vital to exclude breast tissue attenuation artifact in order to make an accurate diagnosis of LAD pathology. Of note, sometimes breast tissue attenuation may coexist with LAD pathology.
5.6.1 Breast Attenuation
Breast attenuation is a frequently encountered artifact 9 (► Fig. 5.2) in A-SPECT and is less apparent with D-SPECT. This artifact typically presents as a fixed perfusion defect in the mid and apical anterior wall and/or anteroseptum on A-SPECT and may appear as a photopenic defect in the middle of the sinogram on D-SPECT (► Fig. 5.3). The main reason a fixed anterior wall perfusion defect is less apparent with D-SPECT may be upright imaging (wherein the breast tissue is located lower down as a result of gravity), high count sensitivity, or a combination of both.


Pearls
Review the rotating projection images on ASPECT to observe for attenuation from breast tissue. 9
When using D-SPECT, breast tissue attenuation is identified by reviewing the rotating projection images as well as the sinograms. Typically, counts are reduced in the midportion of the sinogram (► Fig. 5.3).
Review regional wall motion and wall thickening; if normal, breast attenuation artifact is confirmed. In a prospective study of 99mTc SPECT (and 201Tl SPECT), Taillefer et al showed that concomitant analysis of gated images in female patients improved specificity for CAD detection from 84 to 92%. 16
Most SPECT software packages include quantitative programs that compare patient scans with a normal database, allowing (to some degree) for typical breast attenuation artifact in women.
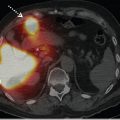
The optimal solution for attenuation artifact is attenuation correction using a radionuclide source or CT transmission scan. If a SPECT MPI study is equivocal due to suspected attenuation on SPECT, a positron emission tomography (PET) MPI study should be considered for further evaluation (► Fig. 5.4). 15 , 17 , 18

Pitfalls
Lack of recognition of breast attenuation artifact may result in an incorrect diagnosis of an infarct in the location of the perfusion defect.
Depending on body habitus and the position of the breast, the lateral wall can also be involved.
If the breast position shifts between rest and stress acquisitions, as identified on the rotating projection images, there may be an apparent reversible perfusion defect mimicking LAD ischemia. Gated SPECT cannot help in this situation. Attenuation-correct MPI is recommended when shifting breast tissue attenuation is suspected.
Small or nontransmural scars (< 50% scar on cardiac magnetic resonance imaging [CMR]) may have preserved wall motion on gated SPECT and may not be identified by SPECT MPI. In one study, 13% of patients with nontransmural scar on CMR had normal rest 201Tl SPECT MPI. 19 In animal models of MI, with histological gold standard comparison, both cardiac SPECT and CMR identified all segments with transmural scar (> 75%); however, small and nontransmural scar (> 50%) was better identified by CMR (92 vs. 28% of the segments). 19
Prone imaging aggravates anterior wall attenuation artifact and is not recommended when troubleshooting possible breast attenuation.
5.6.2 Myocardial Scar and Hibernating Myocardium
Fixed defects in the anterior wall with associated impairment of regional wall motion and thickening typically represent a true abnormality, such as myocardial scar (► Fig. 5.5) or hibernating myocardium (► Fig. 5.6).


Both of these entities are characterized by abnormal wall motion and wall thickening. Myocardial metabolic imaging with fluorine-18 fluoro-deoxyglucose (18F-FDG) PET, however, can differentiate them: the metabolically active hypo- perfused region (mismatch between perfusion and metabolic activity) is hibernating, while the meta-bolically inactive hypoperfused region (matched reduced in perfusion and metabolic activity) is scar. The mismatch pattern is the hallmark of hibernating myocardium and portends an excellent likelihood of recovery of regional function following successful revascularization. Indeed, 18F-FDG PET is the gold standard test to identify myocardial hibernation 20 and can be considered when the percent peak radiotracer uptake is between 40 and 60% 21 and the patient is a potential candidate for coronary revascularization. 22 A detailed description of myocardial viability assessment with 18F-FDG PET is beyond the scope of this chapter, and readers are referred to other excellent reviews on this topic. 22 When 18F-FDG cardiac PET is not available, 201Tl SPECT may be used to assess myocardial viability (see Case 2 in Chapter 11), although it is less accurate than PET.
Pearls
Defect size and severity are similar at rest and stress.
Hibernation or scar may coexist with peri-infarct ischemia in the same or in another coronary distribution.
The gated images demonstrate a regional wall abnormality in both infarcted and hibernating territories.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree