6 Inferior Vena Cava Filters
Summary
Inferior vena cava (IVC) filters are commonly employed in contemporary medical practice in the management of patients with deep venous thrombosis (DVT), often in the setting of pulmonary embolism (PE). There is increasing controversy regarding the appropriateness, safety, and efficacy of IVC filters, and there is a great deal of attention currently on determining what specific patient populations benefit from their use. This chapter outlines the historical data for IVC filters and discusses details of patient evaluation for appropriateness. The chapter is accompanied by a detailed discussion of a complex patient presentation, and the decision-making and management inherent in caring for complex DVT patients are discussed.
6.1 Introduction
Venous interruption has been employed as a means to prevent fatal pulmonary embolism (PE) since the late 18th century when John Hunter performed the first femoral vein ligation. 1 , 2 Bottini reported the first successful interruption of the inferior vena cava (IVC) for prevention of fatal PE in 1893. 1 , 3 Over the next 60 years, open surgical ligation was the preferred method of IVC interruption, despite an overall mortality rate of 14%, recurrent PE rate of 6%, and a fatal recurrent PE rate of 2%. 1
In an effort to reduce the mortality rate associated with surgical ligation, as well as improve upon the morbidity of IVC interruption, IVC plication using various staples and clips was developed. This technique improved patient morbidity outcomes modestly, but still carried an overall mortality rate of 12%. Given the persistently high mortality rate, early innovators in the late 1960s began to explore complete and partial IVC-interrupting intraluminal techniques as means to prevent fatal PE. Many intraluminal devices, including detachable occlusion balloons, temporary sieve catheters, and intraluminal clips, were developed.
The most popular device to arise from that early intraluminal experience was the Mobin-Uddin umbrella (Fig. 6.1). The Mobin-Uddin umbrella was intended as a permanent endovascular plication device that would gradually interrupt the IVC via occlusion. Two drawbacks to the design were: (1) because the original umbrella was 23 mm in diameter, it was too small to prevent occasional cranial migration, including embolization to the heart, and (2) development of thrombus within and above the device often caused caval thrombosis and allowed recurrent PE.
The Greenfield filter, introduced in 1973, was designed for operative insertion from either the internal jugular or common femoral vein approach. 1 It was not until a decade later that the first endovascular placement of a Greenfield filter was described, using a 28-French sheath via a transjugular approach. 4
The Greenfield filter ameliorated the problems inherent in earlier intraluminal devices in that its conical shape allowed continued patency of the IVC and decreased the incidence of cranial migration.
Over the past 40 years, many filters have been introduced. To date, there is no published comparative study to demonstrate superiority between them. 4 The FDA approved retrievable filters in the early 2000s. Although the number of IVC filters placed in the United States has dramatically increased over the past 10 years, 5 only two randomized control trials have been conducted to parse out whether IVC filtration in fact does what it is intended to do: prevent clinically significant pulmonary emboli. 6 , 7 It should be noted that even a randomized control trial despite careful planning and protocol may be fraught with bias and lead to erroneous conclusions. For instance, the clinical trials by Decousus et al and Mismetti lacked groups consisting of patients with acute deep venous thrombosis (DVT) or PE who received only IVC filters. Hence, patients who did not have a contraindication to anticoagulation received caval filtration. Thus, patients without an accepted indication for caval filtration received filters under the study protocol. That all subjects in both groups in both studies received anticoagulation compromised demonstration of any benefit of IVC filtration, limiting the real-world application of their findings.
Although no randomized control trial has been conducted for the purpose of evaluating retrievable filters, multiple prospective clinical studies evaluating the safety and efficacy of individual retrievable filters have been published. 8 Because currently published data are inadequate to demonstrate safety and efficacy of the filters currently available in the United States, the PRESERVE study, a prospective, multicenter trial of the majority of filters in current use in the United States, was begun in late 2015 (preservetrial.com, ClinicalTrials.gov identifier NCT02381509). Enrollment of 1,430 subjects in that trial was completed on March 31, 2019. Until a more definitive scientific statement can be made regarding the efficacy of filter placement, it is up to the interventionalist to screen patients appropriately and place filters according to guidelines such as those set forth by the Society of Interventional Radiology (SIR) Multidisciplinary Consensus Conference. 9 This chapter was written with those guidelines in mind and can be used as a best practice guide when considering a patient for IVC filter placement.
6.2 Case Vignette
6.2.1 Patient Presentation
A 29-year-old woman returned to longitudinal interventional radiology clinic for evaluation for IVC filter removal, 3 months following placement of the filter.
She had undergone catheter-directed thrombolysis for symptomatic left iliofemoral DVT as a result of May–Thurner syndrome (discussed elsewhere in this volume), with concurrent left common iliac vein angioplasty and stenting. Due to rethrombosis 3 days following her procedure, while therapeutically anticoagulated with enoxaparin, repeat thrombolysis was performed. After a discussion of risks, benefits, indications, and alternatives to IVC filters, an IVC filter was also placed. Risks specific to the IVC filters that were discussed prior to placement included migration, fracture caval wall penetration, embolization of all or a portion of the filter, recurrent DVT, IVC thrombosis, and PE. The indication for IVC placement was recurrent DVT while on anticoagulation (Table 6.1).
Past medical history was significant for multiple miscarriages, and her DVT event, her first, had followed a recent miscarriage. During evaluation of her DVT, a thrombophilia panel was performed, and was positive for antiphospholipid antibodies.
Since her repeat thrombolysis and placement of the IVC filter, she had been therapeutically anticoagulated with warfarin. She had returned to the office for 1-month follow-up and at that time complained of intermittent left lower extremity pain, but denied swelling. She was tolerating exercise, but had not yet returned to work because of pain with prolonged standing. At the time of the 1-month office visit, duplex ultrasound of the left lower extremity demonstrated patent deep venous system with small residual, nonocclusive thrombus within the common femoral vein. It was determined at that stage to continue filtration, with a return for reevaluation in 2 months.
At the time of 3-month follow-up, she reported improved symptoms and increased activity. A duplex ultrasound of the left lower extremity was unchanged from the prior. Given her stability on warfarin, it was decided to schedule her for venography to include evaluation of the iliac stent and IVC filter retrieval.
6.2.2 Physical Exam
No left lower extremity edema or tenderness to palpation, no erythema. Distal pulses are equal and intact bilaterally.
6.2.3 Labs
Complete blood count and comprehensive metabolic panel are within normal limits; international normalized ratio (INR) is 2.5.
6.2.4 Imaging
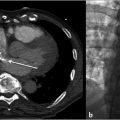
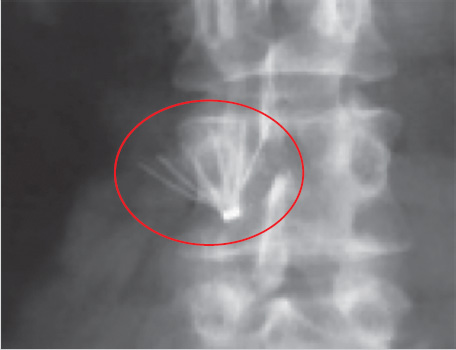
Prior CT was reviewed to evaluate for any issues pertaining to the filter. It was noted that the apex of the filter was tilted posteriorly and likely abutting the posterior cava wall (Fig. 6.2).
6.2.5 Specifics of Consent
Prior to the attempt to retrieve the filter, a thorough conversation with the patient included the risks and benefits of both maintenance and retrieval of the IVC filter. She was advised of the absence of data supporting long-term filtration, but of the theoretical risk that removal of the filter could permit PE should she develop unstable DVT in the future. She was also advised of the risks of long-term presence of IVC filters, including IVC thrombosis, filter fragmentation, and migration of the filter or fragments, as well as strut penetration with vascular injury, bowel injury, or pain. She was also advised that the incidence of these complications was unknown. With respect to risks of the retrieval procedure, the risks of sedation were reviewed, as well as the risks of vascular access, contrast use, and prolonged fluoroscopy. Finally, the risks of failure of retrieval, of caval injury including life-threatening extravasation, and of inadvertent damage to the filter with periprocedural embolization of the filter or fragments were reviewed. Various techniques and tools that might be used during the procedure were also reviewed, with the likelihood of use of devices off-label should the need arise.
6.2.6 Details of Procedure
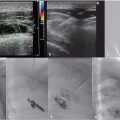
Access was achieved via ultrasound-guided right internal jugular vein puncture and a 5-French pigtail catheter was advanced to the left common iliac vein for angiographic evaluation of the stented left common iliac vein and the IVC. The external and common iliac veins (including the stent) were widely patent without evidence of thrombus. Caval venography demonstrated no abnormality. A 14-French braided sheath was advanced to the superior IVC, through which a 2.5-cm loop snare was placed for retrieval of the filter.
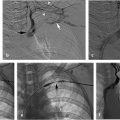
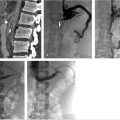
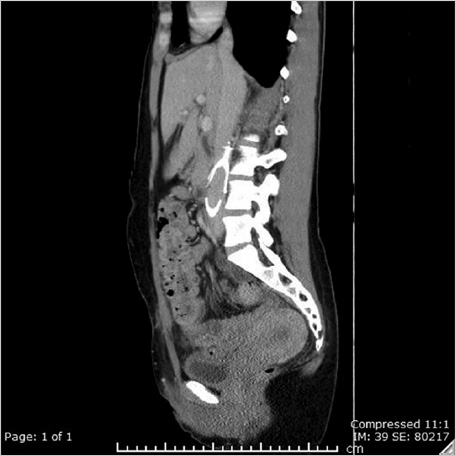
Despite multiple attempts using different snares, the apex could not be snared, as it was likely embedded in the posterior wall of the IVC. A reverse-curve catheter was advanced over a wire into the caudal IVC. A hydrophilic wire was advanced through the catheter, then back up through the legs of the filter so that the wire could be snared above the filter (Fig. 6.3a). Once the wire was snared, it was pulled through the sheath so that both ends of the wire were accessible at the neck. The reverse-curve catheter was removed and the wire was clamped with a hemostat such that the wire was one cohesive unit, allowing application of back tension during the oversheathing of the filter. With tension on the wire, the retrieval sheath was advanced to the apex of the filter. The apex was partially dislodged from the caval wall but not enough to pull the apex into the sheath (Fig. 6.3b). To fully align the apex with the sheath, the loop snare was readvanced through the sheath, alongside the looped wire, and the retrieval hook was snared in the conventional fashion. Tension was maintained on the wire during this maneuver to keep the apex in a reasonable position for snaring. The apex was snared, and the retrieval sheath was advanced to the apex, which was over-sheathed (Fig. 6.3c) beyond the filter legs to free the filter and allow for complete removal. The legs of the filter readily released and the filter was removed in its entirety. A pigtail catheter was advanced over a wire into the caudal IVC and postretrieval venography was performed. There was no evidence of injury to the IVC. The catheter and sheath were removed and hemostasis was obtained with manual compression. She was monitored for 2 hours and was subsequently discharged from the department.
Follow-up
The patient was seen at 1, 3, 6, and 12 months in clinic and was doing well and instructed to follow up as needed with interventional radiology. She will undergo lifelong anticoagulation managed by the hematology service.
6.3 Epidemiology and Scope of the Problem
Venous thromboembolism (VTE) accounts for 250,000 hospitalizations annually in the United States. VTE is a major contributor to in-hospital mortality, with rates as high as 30%. Mortality rates are lower among patients with idiopathic venous thrombosis and highest in the setting of cancer. With the arrival of anticoagulants, VTE can be effectively treated. Given the high mortality rate and readily available treatment, anticoagulation is the standard of care in patients without contraindications. 10 In the United States, it is estimated that first-time VTE occurs in approximately 1 per 1,000 persons per year with approximately one-third of cases PE and two-thirds of cases DVT. Rates increase markedly after the age of 45. In patients over 80, incidence increases to 5 cases per 1,000. Risk factors thought to coincide with increasing incidence with age include venous valve incompetence with increased venous stasis, sedentary lifestyle, and acquired comorbidities. 10 , 11 Rates are slightly higher in men than women. Outcomes of VTE include death, recurrence, postthrombotic syndrome, and bleeding secondary to anticoagulation.
Given the increasing trend of placing prophylactic filters in patients who are deemed high risk for PE, an understanding of the risk factors for venous thrombosis is necessary. Major risk factors for DVT are shown in Table 6.2. To mitigate risk of VTE, patients should be risk stratified and modifiable risk factors should be addressed. Thromboprophylaxis should be initiated when deemed appropriate. Half of all VTE in a longitudinal study looking at 21,680 people over a 7-year period were considered secondary to triggering risk factors. Important triggering risk factors that may prompt an IVC filter consultation include hospitalization, cancer, surgery, and trauma.
Age Surgery Hospitalization Immobility Trauma Pregnancy Oral contraception | Cancer Obesity/overweight Inherited coagulopathy Acquired coagulopathy |
Prophylactic filter consults for surgical and trauma-related indications have significantly contributed to the sharp increase in IVC filter placements in the United States. 12 It is unclear whether a filter in that population is of any benefit. There is evidence suggesting prophylactic filter placement has no effect on reducing trauma patient mortality, while increasing the incidence of DVT. 13
IVC filter placement has increased 25-fold in the United States in the past 20 years and 3-fold from 2001 to 2006. 14 Whether increased filter usage has, on the whole, been of benefit to patients is unclear. Guidelines from two societies, the SIR 8 and the ACCP, 15 differ in their recommendations, both with little level 1 data to support their positions. Further, multiple studies have shown poor compliance with both guidelines, with up 50% of filters placed without a clear indication. 16 Inappropriately placed IVC filters put undue risk on patients, and the cost to society can be tremendous. Therefore, strict adherence to the SIR or ACCP guidelines should be the standard of care until better level 1 data are available. Broadly accepted indications include lower extremity DVT in the setting of an absolute contraindication to, or documented failure of, anticoagulation therapy. 16 The SIR has added prophylactic filter placement guidelines to their recommendations. However, these cases should be limited as much as possible as a true benefit has not been shown to date. If a filter is placed for prophylactic reasons, diligence must be taken to ensure that 100% of these filters are removed once the “at-risk” period has passed.
6.3.1 Patient Presentation and Evaluation
When considering a patient for IVC filter placement, one should adhere to the accepted absolute indications as recommended in the SIR guidelines including: (1) failure of anticoagulation, (2) complication while on anticoagulation, or (3) contraindication to anticoagulation.
The other common reason IVC filtration is requested is for short-term prophylaxis against potential PE in high-risk patients for VTE undergoing surgery. This indication, and filter placement status post trauma, are thought to be in large part responsible for the large increase in IVC filter utilization. Although there has been no proven benefit in placing filters for that reason, it has become an acceptable indication in most institutions by proxy rather than by data. The risks and benefits of filter placement should be discussed in detail with the patient prior to the procedure. A retrieval follow-up plan, and the infrastructure to execute that plan, should be in place to ensure retrieval is carried out once the at-risk period ends.
The next consideration is the location and extent of the thrombus. Is it uni- or bilateral? Does the thrombus extend into the popliteal vein? Is there iliofemoral thrombus? Iliocaval? Is there thrombus free floating in the IVC?
In general, if the DVT involves only the veins below the knee, an IVC filter is not recommended. Although there is very little risk for below-knee thrombus to propagate to the pulmonary arteries, close observation for cranial extension within the deep venous system in these cases may be warranted. This risk increases as the thrombus extends cranially within the deep venous system of the leg. If the location or extent of the thrombus is within the popliteal vein or higher and there is an absolute indication for filter placement, the patient may benefit from IVC filtration to protect against clinically significant PE.
Consultations for IVC filter placement for prophylactic reasons are a bit more nuanced. These patients will present with a future contraindication to anticoagulation: impending surgery. However, these patients have yet to develop any DVT and mostly likely never will even despite their high-risk status. IVC filters can be placed safely in this population with little risk. 10 The majority of complications arise when the patients are lost to follow-up and the filters are never retrieved. The 8-year follow-up data from the PREPIC trial demonstrated that although the incidence of PE was significantly lower in the filter group, there was a 37% increased risk of developing DVT in those patients who received IVC filters versus those who did not. It is of utmost importance that these patients are followed up and their filters are retrieved promptly, thereby mitigating the long-term risks of IVC filtration.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree