6 Lung Cancer–Screening Results Reporting
Summary
This chapter discusses the appropriate means of measuring both rounded and ovoid nodules detected on lung cancer–screening examinations as well as other variable morphologic descriptors. The Lung CT Screening Reporting and Data System (Lung-RADS) for the standardized reporting, description, categorization, and management of detected lesions is described. Illustrative case examples of Lung-RADS 1, 2, 3, and 4 lesions and a sample radiologic Lung-RADS report are provided.
Keywords: nodule, nodule descriptors, measurement, Lung-RADS, radiology reports
6.1 Introduction
The American College of Radiology (ACR) Lung CT Screening Reporting and Data System (Lung-RADS) has been produced by the ACR Lung Cancer Screening Committee subgroup on Lung-RADS. This data and reporting system is a quality assurance tool. It has been specifically designed to standardize the reporting, description, categorization, and management of lesions detected on screening computed tomography (CT) examinations. Lung-RADS also alleviates potential confusion in the interpretation of LDCT studies by both radiologists and clinicians and provides a means to monitor outcome among screened individuals. The first component of the Lung-RADS reporting system is a description of nodule appearance using a specific nodule lexicon and a standardized method of nodule measurement based on morphology. The second component involves the actual categorization of the detected lesion(s) on a scale of 1 to 4 based on morphologic appearance, probability of possible neoplasia, and management recommendations. The last component addresses significant incidental pulmonary or nonpulmonary findings that may impact patient care and management (Chapter 7).
6.2 Nodule Parameters and Measurement
It is not uncommon for many individuals undergoing LDCT screening to have one or more subcentimeter nodules or nodular opacities detected. We suggest that in such instances the six most ominous nodules should be formally reported by the radiologist according to the following descriptors: size, density, presence, or absence of calcium, pattern of calcification if present, presence of fat if present, shape, morphology, and location in the data set (image slice, series, and plane; ▶ Table 6.1). Although, by convention, most nodules should be described based on lung windows in the axial plane, some lesions may be better delineated or characterized on sagittal or coronal planes and can be utilized accordingly. These descriptors are extremely important in the follow-up analysis of lesions to assess stability or interval change. Although not mandated, volumetric lung nodule analysis utilizing currently available software for solid nodules is encouraged and will likely become the standard in years to come. We currently advocate volumetric lung nodule analysis for nodules ≥ 6 mm in diameter. If a given patient has additional indeterminate subcentimeter nodules on their baseline-screening exam (i.e., more than six), a generalized statement can be made in the formal report as to their presence, average size or size range, and morphology. These latter nodules also need to be carefully analyzed on follow-up studies for changes in growth, number, morphology, and/or attenuation.
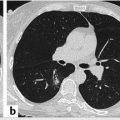
By convention, if the solid or ground-glass nodule is round or spherical, a routine bidirectional (point A to point B) measurement of its diameter on lung window and level settings should be reported (▶ Fig. 6.1). Alternatively, the average diameter should be reported for ovoid or lentiform nodules (▶ Fig. 6.2). As previously discussed in Chapter 5, the radiologist should record the total diameter of part-solid nodules as well as the diameter of its solid component. The solid component should always be measured on mediastinal window and level settings.


6.2.1 Lung CT Screening Reporting and Data System (Lung RADS)
The ACR developed the “Lung-RADS” system for the specific reporting, follow-up, and management of both negative and positive LDCT screens. The first edition of the Lung-RADS classification scheme was modeled after the “BI-RADS” (Breast Imaging-Reporting and Data System), now in its fifth edition, long utilized for reporting screening mammography results. Like BI-RADS, Lung-RADS scores or categories are determined largely by the dominant lesion size and morphology. The size threshold for an actionable nodule or positive screen applying Lung-RADS is ≥ 6 mm for solid and part-solid nodules and ≥ 20 mm for nonsolid (ground-glass) nodules. On follow-up screening CT exams, the size cutoff is ≥ 4 mm for solid and part-solid nodules and/or an interval growth of ≥ 1.5 mm of preexisting nodule(s). New or growing nonsolid nodules must meet the ≥ 20-mm size threshold to be considered positive.
Five potential numerical Lung-RADS categories (0–4) may be assigned when reporting nodules on screening LDCT. Three modifiers (“X,” “C,” and “S”) can also be added to any one of these numerical categories if findings other than nodules are present. The “category number” plus the “modifier code” creates the final Lung-RADS score. The “X” modifier may be used when there are additional imaging findings such as spiculated borders, a rapidly enlarging ground-glass nodule with a doubling time less than 1 year, or enlarged lymph node(s) are seen. The “S” modifier denotes the presence of additional potentially clinically significant incidental findings (e.g., coronary artery disease, emphysema, aorta aneurysm, etc.; Chapter 7). The “C” modifier applies to those screening individuals with a prior established diagnosis of lung cancer returning for follow-up for screening. It may be added to any Lung-RADS category.
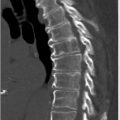
Lung-RADS 0 is rarely applied in those cases in which the screening exam could not be fully completed or one in which preexisting CT studies exist but are not available to the radiologist at the time of the initial screen interpretation. This score is then modified accordingly once the study is completed or those antecedent studies become available to the radiologist for direct correlation. Lung-RADS 1 is essentially a negative screen. That is one in which no nodules are seen on the screening exam or one that demonstrates nodules with distinctly benign patterns of calcification (e.g., completed calcified, peripheral egg shell calcifications, popcorn kernel-like central calcifications, or internal fat; ▶ Fig. 6.3). Lung-RADS 2 is also considered a negative screen, demonstrating nodules that have a very low likelihood of becoming a clinically active cancer due to their size, morphology, or lack of growth on follow-up studies. Both Lung-RADS 1 and Lung-RADS 2 screen results are followed annually with repeat LDCT as long as the individual remains eligible. Lung-RADS 3 studies are likely benign revealing nodules with a low likelihood of becoming a clinically active cancer but warrant closer surveillance with short-term follow-up imaging over 3 to 6 months for reassessment. Lung-RADS 4 is a suspicious screen with findings representing potential lung cancer until proven otherwise. These latter cases often warrant further additional diagnostic testing (contrast-enhanced chest CT, positron emission tomography [PET]/CT) and or tissue sampling. Lung-RADS categories 0 to 4 are summarized in ▶ Table 6.2. In the vast majority of screening studies, 90% of individuals will receive a score of Lung-RADS 1 or 2. About 4% of individuals will receive a score of Lung-RADS 3 and roughly 4% a Lung-RADS 4 score (▶ Table 6.3). Lung-RADS 4 is further stratified into the following: Lung-RADS 4A—solid nodules ≥ 8 to < 15 mm or part-solid nodules ≥ 8 mm with solid component ≥ 6 and < 8 mm; Lung-RADS 4B—solid nodules ≥ 15 mm or part-solid nodules with solid component ≥ 8 mm; and Lung-RADS 4X, which basically represents either category 3 or 4 nodules with additional features suspicious of malignancy (e.g., lymphadenopathy). The nodule with the highest individual Lung-RADS score ultimately defines the final assigned Lung-RADS category. “S” and “C” modifiers may also be applied to any Lung-RADS score on initial baseline and follow-up screens. A comprehensive breakdown of the Lung-RADS categories, description, imaging findings, management recommendations, probability of malignancy, and estimated prevalence in the screening population is provided in ▶ Table 6.4, and is also directly available through the ACR at their Web site (https://www.acr.org/~/media/ACR/Documents/PDF/QualitySafety/Resources/LungRADS/AssessmentCategories.pdf).

Expected distribution (%) | Probability of malignancy (%) | Lung-RADS |
90 | < 1 | 1 or 2 |
4 | 1–2 | 3 |
2 | 5–15 | 4A |
2 | > 15 | 4B |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree