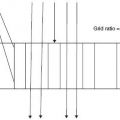
Milne and colleagues110 demonstrated that one can obtain a large amount of useful hemodynamic information from consideration of the vascular pedicle width (VPW).
VPW is often important in determining the etiology of pulmonary edema. Milne’s group111 also demonstrated that using VPW combined with the increased cardiac thoracic ratio is the most helpful sign in elucidating the etiology of the patient’s problem. The vascular pedicle width is measured by dropping a perpendicular line from the edge of the left subclavian artery at its junction with the aorta and then connecting this with a horizontal line across the cardiac shadow to a point just distal to where the superior vena cava crosses the right main stem bronchus. One must remember that vascular pedicle width gives us an estimation of intravascular volume status. This width should be 7–8 cm. The cardiothoracic ratio is evaluated by dividing the widest transverse diameter of the cardiac silhouette by the widest transverse diameter of the thorax, just above the diaphragm. Anything above 50% represents cardiomegaly.
Normally there is a balance between the capillary pressure and the plasma oncotic pressure, so the alveolus remains free of fluid. (The plasma oncotic pressure is the pressure exerted by the proteins.) The integrity of the capillary wall is important in maintaining this normal homeostatic mechanism. Damage to the membrane allows fluid and proteins to enter the interstitium of the lung, thus changing pressure relationships. The lymphatics of the lung play a critical role in eliminating fluid from the interstitum, and they can be discerned on the chest radiograph when filled with fluid. Normally, the flow across the capillary membrane is determined by the Starling equation. From the interstitium of the lung, fluids and proteins are picked up by the lymphatic system and brought back into the bloodstream. The capillary pressure drives fluids across the capillary membrane into the interstitium when this pressure exceeds 8 mm Hg. The plasma colloid osmotic pressure causes fluids to move back from the interstitium into the pulmonary capillary. Normal plasma oncotic pressure exerted by plasma proteins is approximately 25 mm Hg, ensuring a normal flow of water back out of the extravascular space into the capillaries. Pulmonary edema can result when either the hydrostatic pressure has increased or, occasionally, when the colloid osmotic pressure is markedly decreased. This decrease in plasma proteins has to be quite significant and so is a rare occurrence. On the other hand, pulmonary venous hypertension is the most common cause of pulmonary edema.
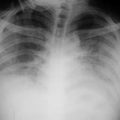
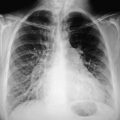
One can fairly adequately determine left ventricular end-diastolic pressure and thus the degree of failure demonstrated on the chest x-ray, if some simple basic principles are followed.112 This often determines how to treat a patient. The first abnormality that occurs is a redistribution of blood to the upper lobe pulmonary veins, socalled cephalization (Fig. 7.2).
Figure 7.2 The film of a patient who was admitted with an acute myocardial infarction reveals the classic appearance of increased blood flow to the upper lobes, cephalization. Note the excellent position of the intraortic balloon pump just distal to the origin of the left subclavian artery.
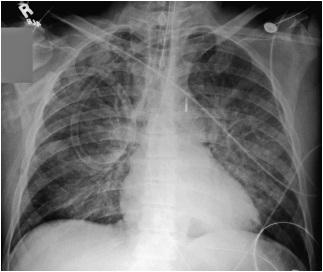
This occurs due to vasoconstriction in the lower lobes and occurs when left ventricular end-diastolic pressure reaches 10-14 mm Hg.
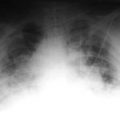
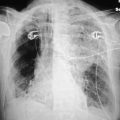
As the pressure in the left ventricle continues to increase, fluid begins to accumulate in the perivascular spaces. There is blurring of the sharpness of the vessels, as there is now fluid between the wall of the vessels and the surrounding alveoli. This so-called perivascular cuffing occurs at a left ventricular end-diastolic pressure somewhere between 14 and 18 mm Hg (Fig. 7.3).
Figure 7.3 A patient with marked cardiomegaly and left ventricular endiastolic pressure elevated to 15-16 mm Hg. Note the increased flow to the upper lobe veins as well the indistinct vascular markings secondary to perivascular cuffing.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree