8 Musculoskeletal Ablation
Pain from skeletal metastases is the most common source of intractable pain in cancer patients. It is the presenting system in nearly 80% of patients with bone metastases.1 In an ambulatory patient group, only 45% described adequate control of pain from medications, and 23% stated that analgesics had no effect at all. This group was otherwise highly functional: 27% still worked, and only 16% considered themselves disabled. The long-term effects of poorly controlled pain from skeletal metastases are undeniable. Over time, mobility and quality of life are diminished, resulting in anxiety and depression. Traditional oncologic therapies provide benefit to many, but not all, patients. Additionally, these historic treatments have a delayed onset of pain relief. Thermal ablation has emerged as a viable option to treat patients with pain from skeletal metastases based on published outcomes, the limited number of treatment cycles required, and the relatively rapid onset of relief. In this chapter, the current status of ablation is compared with outcomes with traditional therapies. Given the emergence of vertebroplasty and cementoplasty, as well as the combined use of these techniques with ablation, the relative role of these options is reviewed as well. Technical tips are provided, as well as guidance regarding patient evaluation and management.
♦ Current Treatment Options
Traditional oncologic therapies have been divided into three major categories: surgical, radiation therapy, and medical. The selected treatment option for a given patient varies depending on the type of malignancy, the number of tumors, and their location.
Surgery
Absolute indications for surgery are a point of discussion, although there is agreement that repair or extirpation of painful skeletal metastases is palliative. A variable indication is the expected time of survival, with some practitioners favoring surgery in patients with a potential survival of 4 weeks or greater, whereas others recommend surgery only if minimal expected survival is 3 months.2,3 Accepted historic indications include radiation-resistant disease, spinal instability, spinal cord/nerve compression, acute neurologic deterioration, and either an impending or present pathologic fracture.3,4 A pathologic fracture is defined as a fracture occurring in a bone under the setting of normal physiologic stress.4 Most skeletal metastases develop in long bones. In a series of surgically treated patients, almost 60% of metastases requiring surgery were in the femur alone.3 In this study, the humerus was involved next most commonly (18.8%), followed by the spine (10.2%) and pelvis (5.9%). Skeletal metastases are rarely solitary and are isolated in only 5 to 15% of patients.3
When needed, it is preferable for surgical repair to be performed prior to the development of a pathologic fracture. In long bones, indications include pain as well as osteolytic foci >2.5 cm in diameter or involvement of >50% of the circumference of the bone. Greater than 50% involvement of the cortical circumference is associated with a 50% risk of pathologic fracture.3 Spine surgery is more complex, with most indications surrounding avoiding or treating devastating neurologic complications. Primary objectives include nerve root decompression, spinal stabilization, and reconstruction of the spinal column.4 The approach to spinal reconstruction depends on the involved portion of the column. Tumors isolated to the posterior elements or the anterior three quarters of the vertebral bodies are approached posteriorly or anteriorly, respectively. Tumors involving the posterior quarter of the vertebral body require both anterior and posterior access, a more complex and challenging intervention.4
Most surgical series are retrospective and consist of treatment of metastases from a variety of tumor types in different bones. One series described surgery on 76 pathologic fractures and 41 impending fractures.3 Only 15% of these patients had a solitary metastasis, and 80.4% of the treated areas were in the long bones of the upper and lower extremities. The majority of the remaining patients (16.1%) had tumors in the spine and pelvis. The surgeries were associated with high morbidity. Patients required three to eight units of packed red blood cells, and the average postoperative admission time was 17.8 days. Several patients were not able to bear weight after surgery, a finding that has been described in other studies following hemipelvectomy.5 Over half the patients maintained the same need for narcotic medications following surgery that they had prior to reconstruction.
Katzer et al3 did not categorize complications as major or minor, but instead described “wound” and “systemic” complications, which occurred in 24.8% and 14.5% of patients, respectively. Significantly, 7.9% of patients died in the hospital following surgery. When surgery is performed in the pelvis, spine, and long bones, a wide resection margin is favored.5 If this is not possible, supplemental radiation is typically added to the treatment regimen to decrease the risk of local disease progression. When fixation screws are placed, radiation therapy is often added to the treatment regimen to prevent loosening secondary to progression of disease. One study found a decrease in loosening of spinal hardware from 15% to 3% with the addition of postsurgical radiation. Optimally, surgery is performed first to maximize healing.6
Radiation
Radiation therapy is the most commonly employed therapy for patients with painful metastases. Interestingly, given the common utility of this technique, standard protocols are not established and remain a point of debate without optimally defined delivery methods. External beam radiotherapy (EBRT) is commonly delivered with a target dose reached over several sessions/fractions. The most commonly described schedule is a total of 30 Gy achieved over 10 fractions.7 This fractionation schedule is based on a Radiation Therapy Oncology Group study of 1016 patients in which five different regimens were compared.8 In this study 54% of patients achieved complete relief of pain, and 83% had at least partial relief. The median duration of complete and partial pain relief was 12 and 20 weeks, respectively. A later metanalysis compared various treatment regimens with outcomes. Included in the comparison were single versus multiple fraction treatment plans. Additionally, different target doses in multifraction treatment plans were compared.9 No difference was found when treatment was given in a single fraction versus multiple fractions when partial and complete pain relief were evaluated. Most of the single fraction therapies prescribed a lower total dose. Not surprisingly, patients treated with a single fraction were also more likely to have recurrent pain and require re-treatment than were those treated with multiple fractions.
Prospective studies have since been performed evaluating the benefits of EBRT given in a single versus multiple fractions. A Dutch multicenter trial compared 24 Gy over six fractions to 8 Gy in one fraction. Achieved palliation was equal between groups: 73% of multiple fraction patients achieved pain relief versus 71% of the single fraction group.10 Again, 24% of the single fraction patients who achieved benefit required reirradiation versus 6% of the multiple fraction patients. A separate randomized trial followed patients for a median of 12 months and found no difference in time to first improvement in pain, time to complete pain relief, or time to increase in pain or toxicity with any of three treatment regimens. These regimens included 30 Gy in ten fractions, 20 Gy in five fractions, and a single fraction of 8 Gy.11 Consistent with previous studies, the single fraction group required retreatment more commonly than patients undergoing the other regimens.
External beam radiotherapy is helpful for the majority of treated patients. However, the maximum benefit of relief is often not manifested until up to 4 weeks after treatment.7 Only 50% of patients have any relief of pain at 2 weeks.12 Not all tumors are equally radiosensitive. EBRT is the most successful in treating lymphoma, myeloma, seminoma, and neuroblastoma, with more moderate success when treating breast and prostate metastases.4 EBRT also has several toxicities. Common side effects include fatigue, skin erythema, and nausea. Depending on the location of the field, diarrhea or esophagitis is common. Larger fields, particularly over the pelvis and spine are associated with myelosuppression. This risk is likely elevated in patients pretreated with chemotherapy. Pathologic fractures are a potential risk in 4 to 18% of patients following EBRT.8 The risk is likely greater in long bones than in the spine.7
Bisphosphonates
Bisphosphonates are analogs of pyrophosphate that inhibit osteoclast-mediated bone resorption. A variety of agents have been developed and are principally used in patients with breast cancer and, less commonly, multiple myeloma.13 Breast cancer has been the most commonly studied tumor for several reasons: bone metastases develop in up to 70% of this patient group, and survival of breast cancer patients with skeletal metastases is 25 months, much longer than prostate (6 months) or lung (3 months) cancer.14 Given the high incidence of skeletal metastases in patients with breast cancer and the relatively prolonged survival, 10 to 20% of these patients develop pathologic fractures. Once initiated, bisphosphonates are given for life.
Typically, outcomes with bisphosphonates have been measured by a decrease in skeletal-related events (SREs), which include pathologic fracture, requirement of EBRT for intractable pain, surgery for spinal cord compression, and hypercalcemia of malignancy. The newest bisphosphonate on the market, zoledronic acid (Zometa, Novartis, East Hanover, NJ) is 10,000 times more powerful than first-generation agents and is the first drug in this class to be approved for treatment of skeletal metastases from other primary tumors. In preclinical testing, zoledronic acid had a 41% reduction in SREs compared with placebo.13
Although outcomes with zoledronic acid are promising, bisphosphonate use is not without controversy. Critics point out that evaluation of SREs does not include measuring or evaluating for changes in the level of pain.14 Other points of contention include that a decrease in SREs with bisphosphonates is not noticeable until 3 to 6 months after the initiation of therapy, and one study found that the cost of the drug being evaluated exceeded the cost savings from the SREs that were prevented. This time to benefit is one reason why this group of drugs was not used for metastasis outside of breast cancer in the past. The most common toxicity with bisphosphonates is osteonecrosis of the jaw.13 When using zoledronic acid, the risk is 1% at 1 year with an increase to 21% at 3 years.
Radionuclide Therapy
Radionuclides are used in the setting of multiple metastases, particularly when EBRT has failed. They are not appropriate for lytic metastases, patients with an impending or pathologic fracture, spinal cord compression, renal insufficiency, or compromised bone marrow function.7 Two agents are most commonly used: strontium-89 and samarium-153.
Strontium-89
Strontium-89 (89Sr) decays by betaemission with a maximum energy of 1.46 MeV, an average soft tissue penetration of 2.4 mm, and a half-life of 50.6 days. 89Sr is taken into the mineral matrix of bone and concentrates at areas of active resorption of skeletal metastases. The retention is proportional to the tumor burden at a given site, and given the long half-life, up to 80% of the administered dose may be taken into the bone by 100 days.15 Patients can experience a pain fare shortly after treatment. When 89Sr was studied versus placebo, 20% and 50% of patients treated with 89Sr reported complete and partial pain relief, respectively.16 The typical administered dose is 1.5 MBq/kg.17 In a study of 83 patients, this dose resulted in some pain relief in 75% of patients and complete pain relief in 22%.17 In this trial, the onset of pain relief was typically 10 to 20 days after injection, the benefit maximum was at 6 weeks, and the duration of benefit was approximately 6 months.
Samarium-153
Samarium-153 (153Sm) emits beta-energy at 0.81 MeV, with a half-life of 46.3 hours. Additionally, this isotope has 28% emission of 103 keV gamma rays.7 Compared with 89Sr, 153Sm has a shorter half-life and a higher dose rate, resulting in shorter dose delivery times: 75% of 153Sm is delivered in 4 days compared with 101 days for 89Sr. 153Sm is chelated to ethylenediamine-tetramethylene phosphonate (EDTMP, which results in preferential localization to osteoblastic areas of bone. Given the natural production of gamma rays, 153Sm can also be used for diagnostic purposes as well. In a randomized trial versus placebo, a 1.0-mCi/kg dose resulted in pain relief in 72% of 32 patients.18 In this study 31% of patients reported complete/marked pain relief, and 43% of patients reporting improvement in pain had persistent benefit at 16 weeks.
Outcomes with Thermal Ablation
Radiofrequency Ablation
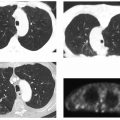
Initial retrospective reports on radiofrequency ablation (RFA) described excellent clinical outcomes. Gronemeyer and colleagues19 used RFA to treat 10 patients with 21 painful spinal metastases. Four patients were treated with supplemental vertebroplasty 3 to 7 days following ablation. Nine of the 10 patients reported a decrease in pain at the time the review was written, with a 74% average pain reduction. A case report by Ohhigashi et al20 described successful thermal ablation of recurrent rectal carcinoma involving the sacrum.
A larger retrospective trial reviewed 34 treatments in 30 patients.21 Using a 10-point pain scale, the authors evaluated the patients’ worst pain, average pain, and mean pain interference with daily life prior to the treatment, and 1 day, 1 week, and 8 weeks following the procedure. They found that 19 of the 30 patients had reduction in pain within 24 hours. At 1 week and 8 weeks, all treated patients had significantly reduced pain and decreased interference with daily life from pain.
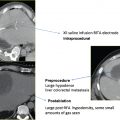
In the largest reported multicenter prospective trial, Goetz et al22 reported outcomes in 43 patients. Patients were followed for 24 weeks after treatment. Prior to ablation, the mean worst pain of the day was 7.9/10. At 4, 12, and 24 weeks after treatment, the worst pain of the day decreased to 4.5/10, 3.0/10, and 1.4/10, respectively. All decreases were statistically significantly improved from the baseline score; 41 of 43 patients experienced significant reductions in pain. Three major complications occurred, all of which should be noted by practitioners performing thermal ablation of skeletal metastases:
A second-degree skin burn at a grounding pad site. Operators performing RFA need to be meticulous about grounding pad placement, ensuring that positioning is appropriate and that there are no gaps in contact with the skin.
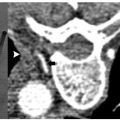
Transient bowel and bladder dysfunction following treatment of a sacral metastasis. Treatment in the sacrum can be associated with motor defects (typically at S1) or, more inferiorly (S2–4), foot drop or bowel and bladder dysfunction. It is critical to discuss these risks with patients and to obtain their signed consent in the interventional radiology (IR) clinic.
A fracture of the acetabulum following treatment of a supraacetabular lesion. Weight-bearing bones are at risk of pathologic fracture when treated with thermal ablation. This potential complication is discussed in greater detail below.
Cryoablation
Callstrom et al23 reported a series of patients with painful skeletal metastases that were treated with cryoablation. Fourteen patients were treated and followed for up to 6 months. Tumors up to 11 cm in diameter were treated. The mean worst pain score prior to ablation was 6.7/10. This rating dropped to 3.8/10 four weeks after treatment. There was also a significant decrease in pain interference with activities of normal living. All patients who required narcotics prior to treatment had a reduced requirement for medication following cryoablation. No major complications occurred. Patients with tumors in weight-bearing areas such as in the prospective multicenter RFA trial were excluded from this study.
Two principal potential benefits of cryoablation were described. One was the ability to visualize the therapeutic ice ball during treatment. This finding is relevant, as the destructive zone created by RFA is not visualized on computed tomography (CT). Operators sometimes see bubbles secondary to cavitation with RFA, but these bubbles do not define the zone of ablation. Another potential benefit of cryoablation was that even though patients treated with RFA often have rapid resolution of pain, the immediate posttreatment period can be associated with temporary worsening. There appeared to be less acute postprocedural discomfort with cryoablation.
Ablation Combined with Cementoplasty/Vertebroplasty
Hoffmann and colleagues24 reported outcomes in 22 patients who were treated with both RFA and cementoplasty/vertebroplasty over a 5-year period. Treated tumors were in the thoracic and lumbar spine, sacrum, pelvis, acetabulum, femur, and tibia. All treated patients had improvement in pain within 24 hours, with a mean worst pain score decrease from 8.5/10 to 5.5/10, a statistically significant reduction. By 3 months, the worst pain of the day had decreased to 3.5/10. The rapid onset of pain relief with combined therapy is notable. Over a mean follow-up of 7.7 months, 15 of the patients had a reduced narcotic requirement. Two patients had an increased narcotic requirement secondary to tumor progression outside the treated area. No major complications were observed.
A study by Munk et al25 reviewed 25 combined treatments in 19 patients. Tumors in the lumbar and thoracic spine, acetabulum, sacrum, pubis, and humerus were treated. Pain was assessed prior to treatment and at 6 weeks after therapy. The mean score changed from 7.9/10 before treatment to 4.2/10 afterward. There were several asymptomatic leaks of cement, and one transient thermal nerve injury when post-procedural CT demonstrated extravasated cement in contact with the sciatic nerve. The patient developed a mixed sensory loss and weakness following the procedure, which resolved almost entirely at 48 hours after treatment.
As treatment options for patients with painful metastases continue to evolve, combination therapy is an intriguing option. Treatment of spinal tumors with thermal ablation is not without risk, as is discussed below (see Anatomic Considerations). Given that skeletal metastases have a propensity to form within the axial skeleton, combined therapy is an appropriate option for some patients to avoid postablation pathologic fracture.26
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree