8 Neoplasms
8.1 Introduction
Neoplasms of the central nervous system (CNS) are the most common solid neoplasms in children and come in a variety of forms. The most common location for pediatric tumors of the CNS is in the posterior fossa, in contrast to those in adults. Adult primary brain tumors are most commonly in the supratentorial brain; however, tumors in children can arise and spread throughout the brain as well as the spine. Many of the most common pediatric primary neoplasms of the CNS can spread via leptomeningeal seeding; therefore, upon detecting them it is imperative to perform contrast-enhanced magnetic resonance imaging (MRI) of the entire neural axis (brain and total spine) to look for disease. Imaging of the spine must extend into the sacrum to include the end of the thecal sac. Although some tumors, such as pilocytic astrocytoma, are not typically metastatic to the leptomeninges, the exact histologic characterization of these tumors depends on their surgical biopsy/resection, and the author therefore believes that all tumors should have an appropriate preoperative workup.
8.2 Tumors Commonly (But Not Exclusively) Found in the Posterior Fossa
8.2.0 Pilocytic Astrocytoma
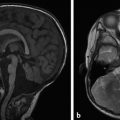
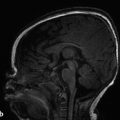
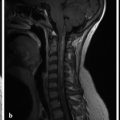
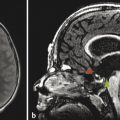
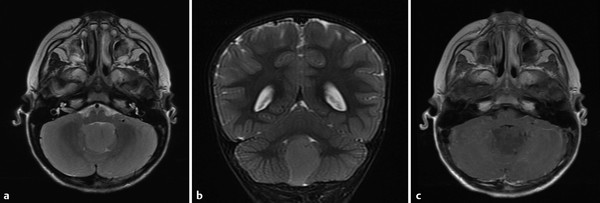
Among the most common pediatric tumors of the brain is pilocytic astrocytoma, often referred to as juvenile pilocytic astrocytoma (JPA). Juvenile pilocytic astrocytomas are World Health Organization (WHO) grade I tumors that are typically managed surgically, and if their gross–total resection is achieved, adjuvant therapy is often not given unless there are suspicious changes on follow-up by imaging. The classic description on imaging of a JPA of the posterior fossa is that of a cystic lesion with an enhancing nodule (Fig. 8.1). The presence of enhancement should not be a dissuasion from recognizing this lesion as a low-grade tumor. The apparent diffusion coefficient (ADC) values of JPAs tend to be high, in the range of 1500 × 10–6 mm2/sec. Although JPAs are intra-axial tumors, they can have exophytic components. And although the cyst-with-nodule appearance of a JPA is very common in the cerebellum, it is less characteristic of pilocytic astrocytomas found elsewhere. Brainstem pilocytic astrocytomas can present as solid enhancing lesions and a discrete brainstem or deep gray-matter lesion without surrounding edema and without low ADC values is nearly always a pilocytic astrocytoma (Fig. 8.2). Gliomas of the optic pathway, most commonly seen in the setting of neurofibromatosis type 1, are also classified histologically as pilocytic astrocytomas, and may represent fusiform thickenings of the orbital segment of the optic nerve or focal exophytic chiasmatic lesions. Most pediatric intramedullary neoplasms of the spinal cord, are pilocytic astrocytomas (further discussed in Chapter 27). Pilocytic astrocytomas have a very low (although not zero) potential for metastatic spread and malignant degeneration. They rarely calcify, and apart from those that occur in the spinal cord they rarely exhibit internal hemorrhage.
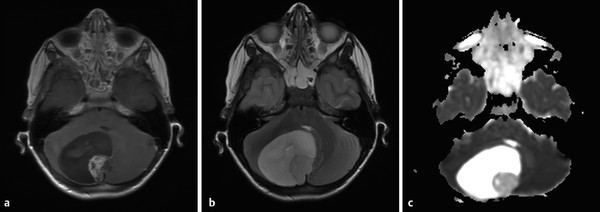
8.2.1 Ependymoma
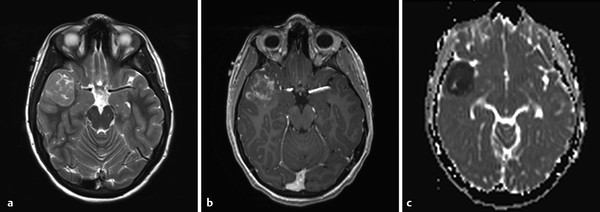
Ependymomas are tumors arising from the ependymal lining of the brain, and in children occur most commonly in the posterior fossa (Fig. 8.3). Ependymomas are usually low-grade (WHO grade II) tumors, with ADC values between those of a JPA and a medulloblastoma. 1 The WHO grade III variant of ependymoma, known as anaplastic ependymoma, is a more aggressive form of ependymoma. Ependymomas of the posterior fossa are most often extra-axial, and can fill the fourth ventricle and extend through one (or both) foramina of Luschka. Once it extends through a foramen of Luschka, an ependymoma can insinuate itself into the lateral medullary cistern and extend superiorly into the cerebellopontine-angle cistern and caudally to (and through) the foramen magnum. The tendency of ependymomas to spread through crevices has been described as “toothpaste-like” spread, and can result in mass effect on, or the encasement of, cranial nerves. Optimal survival after the resection of an ependymoma comes from maximal tumor removal at the time of initial surgery, suggesting that careful attention to the extent of such a tumor (and particularly if it has spread through the basal cisterns) is highly important in helping to guide the neurosurgeon. Adjuvant radiation therapy is used after the removal of an ependymoma. Chemotherapy has not yet proven effective against ependymoma.
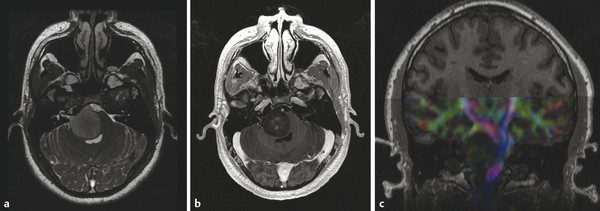
Because ependymomas sometimes demonstrate only minimal enhancement, the evaluation of all imaging sequences of these tumors, as with all tumors, and as opposed simply to post contrast T1 W images, is important. Supratentorial ependymomas are often intra-axial lesions (Fig. 8.4).
8.2.2 Medulloblastoma
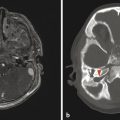
Medulloblastoma (MB) is a high-grade tumor of the posterior fossa, and has more recently been categorized as belonging to a subset of primitive neuroectodermal tumor (PNET), for which reason it is sometimes referred to as PNET–MB. Medulloblastoma can result in obstructive hydrocephalus when it occurs in the fourth ventricle, and it is prone to dissemination through the cerebrospinal fluid. Spinal metastatic deposits of these tumors are also possible, and as pediatric neoplasms of the CNS, their workup should involve a contrast-enhanced magnetic resonance imaging (MRI) study of the brain and entire spine (Fig. 8.5). Although medulloblastoma most commonly occurs within the fourth ventricle, a cerebellar hemispheric variant, known as a desmoplastic nodular medulloblastoma, can occur, and is the most common type of this neoplasm found in older adolescents and young adults. More recent work has classified medulloblastomas on the basis of their genetic profiles in terms of the WNT and Hedgehog genes.
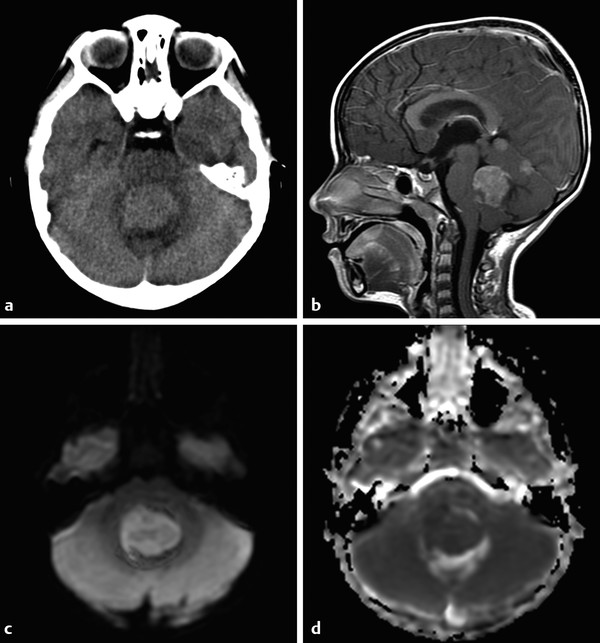
Previously, the presence of calcification had been suggested as a means of differentiating ependymoma from medulloblastoma, but this occurred before the advanced characterization of these tumors with MRI, and is of little practical use in the clinical setting, since both tumors may or may not have calcification. Apparent diffusion coefficient values have proven more effective for attempting to differentiate ependymoma from medulloblastoma than the presence of calcifications, but this alone is not foolproof because anaplastic ependymoma (WHO grade III) can have low ADC values, and atypical teratoid rhabdoid tumor (AT/RT) can have similarly low ADC values. Regardless of histologic characteristics, the ADC values of ependymoma correlate well with tumor grade and cellularity. The high nuclear to cytoplasmic ratio results in both medulloblastoma and AT/RT tumor having appearances of higher density than that of ependymoma on computed tomography, even in the absence of macroscopic calcification.
There are primitive neuroectodermal tumors that are not MB, yet which present as aggressive tumors with low ADC values and a propensity for leptomeningeal spread. Supratentorial PNETs are usually variants of tumors other than medulloblastoma.
8.2.3 Atypical Teratoid Rhabdoid Tumor
Atypical teratoid rhabdoid tumor (AT/RT) is a highly cellular and aggressive tumor that is most commonly seen in the first few years of life. On imaging, and clinically, AT/RT can be difficult to differentiate from MB, and both types of tumor have a propensity for causing leptomeningeal metastatic disease. Atypical teratoid/rhabdoid tumor can be a mass in the fourth ventricle, like medulloblastoma and ependymoma, or an intra-axial lesion.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree