9 Ablation of Esoteric Organs
Although minimally invasive thermoablative therapies have been most extensively described as a means to treat solid organs within the chest and abdomen, clinical studies and experience have suggested a role for percutaneous ablation in a multitude of organs, soft tissue, and skeletal sites throughout the body. As esoteric indications for the use of radiofrequency (RF), microwave, and cryoablation expand to include the adrenal gland, recurrent and advanced malignancies of the head and neck, soft tissue and bony lesions of the pelvis, as well as breast cancers, patients may seek palliation, a minimally invasive alternative to surgical intervention, or treatment for disease previously considered refractory to treatment. With further investigation and creative treatment strategies, thermal ablation will continue to be an integral component of oncology treatment plans as the fourth arm of cancer therapy.
♦ The Adrenal Gland
Patient and Tumor Selection
Primary neoplasms of the adrenal gland include the following: adrenal cortical carcinoma, adenomas (nonfunctioning or cortisol-producing), aldosteronomas, and pheochromocytomas. Traditionally, treatment options have included either open or laparoscopic surgical resection.1–7 The adrenal gland is also the fourth most common site for metastatic disease following the lung, liver, and bone, with contributory primary malignancies including lung, breast, skin (melanoma), kidney, thyroid, and colon cancers.8 The incidence of metastasis is specific to the primary tumor, with approximately 30 to 40% of lung and breast cancers, 50% of melanomas, and 10 to 20% of renal and colorectal malignancies likely to cause adrenal metastases.8 Similarly, surgical resection has been proposed as the treatment of choice for isolated adrenal metastatic disease,9,10 with a median survival of 28 months reported by Sarela et al11 status post-adrenalectomy for isolated adrenal metastasis. Paradoxically, patients with advanced stages of inoperable non-small cell lung cancer metastatic to the adrenal gland have also undergone adrenalectomy with hopes of increasing long-term survival rates.12
Less invasive treatment options such as selective arterial embolization and injection with alcohol or acetic acid have been described.13–16 As the arterial supply of the adrenal gland is threefold in anatomic design, catheterization of these vessels and thorough embolization of the gland remain challenging, and ethanol and acetic acid injections have shown to be insufficient when treating sizable lesions.17 Cryoablation was subsequently presented as a surgical alternative with use documented in both animal studies and in a patient with bilateral adrenal hyperplasia.18 With much literature and clinical practice documenting radiofrequency ablation (RFA) as a safe and effective minimally invasive ablation modality in the liver, lung, kidney, bone, and other organs, a role for RFA in the treatment of adrenal neoplasms was suggested.19–23
Adrenal Cortical Carcinoma
Adrenal cortical carcinoma (ACC), a primary malignancy arising from the adrenal cortex, is relatively rare with an annual incidence of 1 to 2 per 1,000,000 in the United States.24 Radiation and chemotherapy have not proven to be effective treatment options, and thus the mainstay of treatment is surgical resection despite the fact that there is a high rate of local recurrence often requiring repeat intervention.24 Wood et al,25 however, report on the feasibility, safety, and efficacy of using RFA in the treatment of recurrent primary ACC and its metastases in eight patients. A total of 15 lesions were ablated ranging in size from 1.5 to 9.0 cm, demonstrating that RFA was associated with minimal morbidity and was effective in the local control of both recurrent ACC, especially lesions <5 cm in diameter, and its metastases.
Adrenal Metastases
At our institution, percutaneous computed tomography (CT)-guided RFA was initially used 6 years ago to treat 13 solid adrenal masses in 12 patients ranging in age from 40 to 77 years (mean age 58 years).26 All patients were deemed nonsurgical candidates on the basis of medical comorbidities, concurrent disease at distant sites, or refusal of resection. Eleven lesions were metastatic from a variety of primary malignancies (five from lung cancer, four from renal cell carcinoma, and two from melanoma). At the time of RFA, metastatic disease was localized, affecting only the adrenal gland in six of the 11 cases (one patient with bilateral isolated adrenal metastases), and five cases were associated with the presence of additional distal organ metastases successfully controlled by chemotherapy, radiation, or prior resection. Tumor diameters ranged from 1 to 8 cm (mean 3.9 cm).26 To date, clinical experience at our institution continues to show that patients with metastatic disease to the adrenal gland are candidates for thermal ablation, which offers satisfactory local control rates in this population of patients.
Aldosteronomas and Pheochromocytomas
Of the two remaining lesions treated in the Mayo-Smith et al cohort, one patient had been diagnosed with an aldosteronoma and the other with a pheochromocytoma, for each of which laparoscopic surgical resection is the standard therapy, with reported associated morbidity rates of 7.5 to 23%.26–28 It is estimated that in 80% of patients with primary hyperaldosteronism the causative etiology is an aldosterone-secreting adrenal cortical adenoma with autonomous function.24 At presentation, the resulting clinical syndrome of hyperaldosteronism includes symptoms of diastolic hypertension, metabolic alkalosis, and hypokalemia, serving as the cause of ≤1% of clinically diagnosed hypertension.29
In the majority of patients, aldosteronomas are unilateral, affecting a single adrenal gland, and small, with approximately 20% found to be <1 cm, making them challenging to identify on cross-sectional imaging.24 The work of Mayo-Smith and Dupuy26 provides a clinical correlation for these observations. The study patient diagnosed with an adrenal aldosteronoma had clinical hypertension, a 1-cm mass on the left adrenal gland, and elevated serum aldosterone levels that lateralized to the left adrenal at selective venous sampling. This patient required oral potassium supplementation prior to ablation, consistent with a clinical finding of hypokalemia.
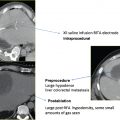
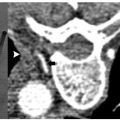
Pheochromocytomas ( Fig. 9.1 ) are rare, hormonally active, neuroendocrine tumors that originate from the chromaffin cells of the adrenal medulla. The excessive secretion of catecholamines, namely adrenaline and noradrenaline, gives rise to clinical symptoms, including headaches, paroxysmal hypertension, diaphoresis, tachycardia, and anxiety. Diagnostic studies include measurement of serum metanephrines and, if necessary, a 24-hour urine collection for catecholamines, vanillylmandelic acid, and metanephrines. Only after a biochemical abnormality is proven with laboratory data should diagnostic imaging be performed.
As a clinical correlate, one of the 12 patients in the Mayo-Smith and Dupuy26 cohort had been diagnosed with a pheochromocytoma after biochemical evaluation, and diagnosis was histochemically confirmed on biopsy prior to ablation. An additional case series by Cheah et al30 documents RFA of a 68-year-old man with a 2-cm right adrenal pheochromocytoma deemed to be a high-risk surgical candidate and a 65-year-old man with a 3.3-cm right adrenal pheochromocytoma with an extensive cardiac history.30 It is standard of care that these patients receive preoperative alpha- and beta-blockade prior to surgical resection, routinely prescribed at our institution in the form of oral phenoxybenzamine and atenolol for 3 weeks prior to ablation, providing alpha- and beta-blockade, respectively.
Anatomic Considerations
The adrenal glands are suprarenal in position lying between T11 and L2, with the right adrenal posterior to the inferior vena cava and the left in close proximity to the aorta and adjacent pancreas. Fatty tissue encases each adrenal, forming an adipose capsule and branching fibrous extensions, which penetrate into the gland, resulting in a relatively fixed anatomic location. The rib cage and lungs extend caudally anterior to retroperitoneal adrenals with several centimeters of overlap. It is noted that during inspiration the distance between the adrenals and kidneys becomes more pronounced.
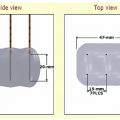
Patients are routinely positioned on the CT gantry bed, depending on the sidedness of the lesion to be treated. The ipsilateral decubitus position was found to be most advantageous when ablating relatively small adrenal lesions. When combined with a posterior percutaneous approach, this position made the adrenal readily accessible while compressing the ipsilateral lung by placing it in a dependent position. Thus, a trajectory could be chosen requiring minimal if any traversement of pulmonary parenchyma. When treating larger adrenal lesions, however, patients are routinely placed in the prone position.
When performing thermal ablation of the adrenal gland, care must be taken to protect the surrounding organs from thermal injury. The kidneys, stomach, pancreas, and liver lie in close proximity to the adrenals and are susceptible to injury, necessitating the use of thermoprotective strategies to isolate the adrenal gland during thermal ablation. For example, hydrodissection requires a 5% dextrose solution to be instilled adjacent to the adrenal gland by means of a CT-fluoroscopically placed catheter until vulnerable, surrounding structures are adequately displaced.31 Additional reported techniques utilize carbon dioxide in a similar manner.32
Technique
As the adrenal gland actively produces and stores catecholamines as well as cortisol, androgens, and mineralocorticoids, ablation of this gland poses the risk of releasing large amounts of both stress and steroid hormones into the bloodstream in a short period of time. Sudden exposure to high levels of circulating catecholamines places the patient at risk of developing rapid-onset tachycardia and hypertension. Thus, patients may be prophylactically treated with alpha-and beta-blockade prior to adrenal ablation.
Despite the fact that pharmacologic blockade is the standard of care prior to ablation of a pheochromocytoma, a controversial debate arises regarding pretreatment of patients who are to undergo ablation of adrenal metastases. Most interventional radiologists do choose to pretreat, as there is no scientific way to predict preprocedurally which patients are most at risk for an intraablative hypertensive crisis.24 At our institution, patients scheduled to undergo ablation of adrenal metastases are premedicated with phenoxybenzamine for 2 weeks prior to the procedure in an effort to provide alpha-adrenergic blockade. Pharmacologic beta-blockade may be simultaneously required in some patients due to resultant alpha-blockade-induced tachycardia, and thus is routinely prescribed in the form of atenolol.
The standard departmental RFA protocol is followed in the treatment of patients undergoing adrenal ablation. All patients routinely undergo staging CT of the chest, abdomen, and pelvis prior to ablation as part of the pretreatment outpatient workup. Neither prophylactic nor intraprocedural antibiotics are routinely administered. Percutaneous ablation treatments are performed under conscious sedation administered by a dedicated nursing professional who continuously monitors electrocardiogram (ECG) tracings and vital signs. If deemed necessary, general anesthesia is used with continuous blood pressure monitoring via an arterial line. In cases at our institution in which a pheochromocytoma is to be ablated, pharmacologic sedatives are administered by an anesthesiologist with prior experience in caring for patients undergoing surgical resection of such lesions.26 Intraprocedural hypertension is a concern, and is reported by Cheah et al30 in a patient who required intravenous nitroprusside during ablation.
Based on lesion size, three-dimensional configuration, location, and proximity to or involvement of neurovascular structures, the most suitable ablative modality, RF, microwave, or cryoablation, is used. When using an RF system, a deployable array, cluster electrode, or switching controller is selected for use, enabling the creation of a larger ablation zone while reducing the number of applications required per session. (An application or treatment is the placement of electrodes and application of RF energy, and a session is a patient’s visit to the radiology suite for an ablation procedure.) For all adrenal lesions >3 cm in diameter, we prefer to use either microwave or cryoablation. With the flexibility of using multiple antennae/probes simultaneously in customized three-dimensional arrays, improved coverage of relatively larger lesions is made possible.
Postablation Management, Efficacy, and Follow-Up
The first several patients to undergo percutaneous adrenal RFA at our institution were admitted for overnight observation due to a lack of clinical data on procedural tolerability.26 As there were no major complications or episodes of post-ablation hypertension, the majority of ablations thereafter were performed on an outpatient basis. Patients were discharged postprocedurally in accordance with the standard departmental protocol. Postablation discomfort was treated with acetaminophen or acetaminophen/hydrocodone. Patients were routinely seen for outpatient follow-up within 2 weeks. Contrast-enhanced CT scans were to be performed at 3- and 6-month intervals from the date of ablation to rule out the presence of residual or new adrenal enhancement indicative of residual or recurrent disease, respectively.
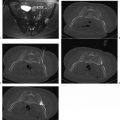
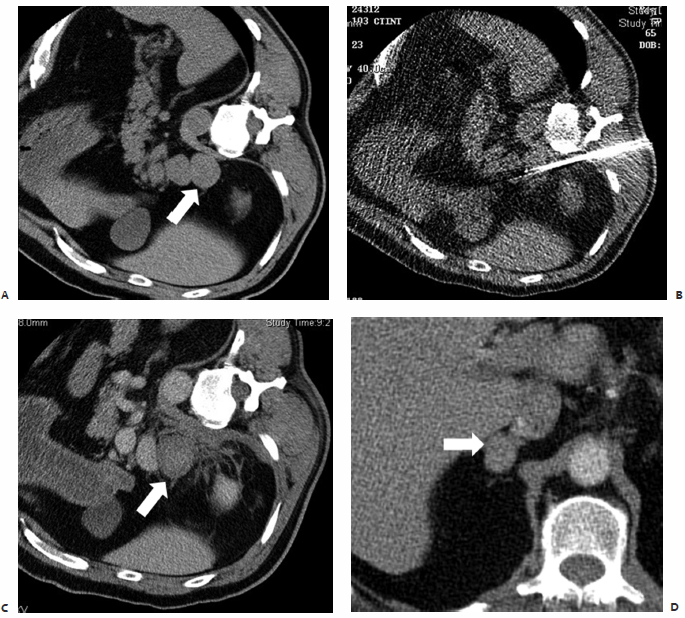
In the study by Mayo-Smith and Dupuy,26 patients were followed for an average of 11.2 months (range, 1 to 46 months) during which time 11 of 13 treated lesions, 85%, revealed neither clinical nor radiographic evidence of residual or recurrent disease, thus indicating complete tumor necrosis and no need for re-treatment ( Fig. 9.2 ).26 A remaining two of 13 lesions were found on follow-up imaging to demonstrate small areas of residual enhancement within the ablation zone, consistent with residual disease. The average diameter of these index lesions was 6.0 cm compared with an average of 3.6 cm among the aforementioned 11 masses. Although not found to be statistically significant, larger lesions were thought to be associated with a higher incidence of residual disease, as the available technology at the time of this study allowed for the treatment of a lesion only ≤5 cm in each single application.26
Similar efficacy of RFA in the treatment of adrenal metastases was shown in Italy. Carrafiello et al17 percutaneously ablated seven adrenal lesions (including the re-treatment of a single lesion with residual disease) ranging in size from 1.5 to 4.0 cm with an average of 2.9 cm. Only one patient developed severe intraprocedural hypertension requiring pharmacologic treatment with beta-blockade. Similar to the outcome at our institution, five of the six lesions revealed no evidence of residual disease on follow-up imaging, and effective local control was demonstrated, especially in those patients with lesions <5 cm in diameter.17
The potential biochemical sequelae of adrenal RFA were illustrated by the patient with bilateral solitary adrenal metastases who underwent two separate ablation sessions at our institution.26 Prior to initial ablation and after treatment of the left adrenal lesion, this patient underwent adrenal function tests. Cosyntropin stimulation tests and renin levels were normal on both occasions, signifying normal glucocorticoid and mineralocorticoid function. Following ablation of the right adrenal lesion, these tests were again performed and found at this time to be significantly abnormal, indicative of thorough ablation of all adrenal parenchyma. Prior studies have estimated that more than 90% of all adrenal tissue must be rendered nonfunctioning before biochemical function is compromised.33 Thus, this patient was pharmacologically managed with oral glucocorticoid (prednisone) and miner-alocorticoid (fludrocortisone acetate) replacement therapy.
Laboratory profiles should be routinely monitored following RFA of biochemically functioning tumors. Serum hormone levels and abnormal biochemical activity are expected to normalize by 1 week, provided that the lesion was successfully treated.24 Serum potassium levels should be closely followed after aldosteronoma ablation. Mayo-Smith and Dupuy26 showed that after RFA of an adrenal aldosteronoma, serum aldosterone levels normalized, and the patient was able to discontinue the use of all oral potassium supplements as no clinical evidence of hypokalemia remained. Similarly, the patient with a biopsy-proven pheochromocytoma was able to discontinue the use of all pharmacologic antihypertensives following ablation and remained normotensive. In correlation with this clinical finding is the lack of significant enhancement of the adrenal mass seen on 1-month follow-up CT images. Importantly, all antihypertensive medications in those patients undergoing ablation of an aldosteronoma or pheochromocytoma should be continued in the immediate postablation period and then gradually tapered under medical supervision.
♦ The Thyroid Gland
Patient and Tumor Selection
The American Cancer Society estimated that there would be over 37,000 new cases of thyroid cancer in the United States in 2009. Although thyroid cancer has a low associated mortality and a collective 5-year survival of 97%, approximately two thirds of patients are between the ages of 20 and 55 at the time of diagnosis.34 To date, the most common subtype of well-differentiated thyroid cancer remains papillary carcinoma, accounting for 80% of all thyroid malignancies. The natural history of papillary carcinoma is that of slow-growing lesions commonly localized to a single lobe of the thyroid gland, with occasional bilobar involvement and frequent metastases to lymph nodes within the neck.34
The standard treatment of papillary carcinoma is surgical resection of the thyroid gland. Either a total or subtotal thyroidectomy is performed, with resection of all suspicious lymph nodes within the central compartment of the neck. If metastasis to lymph nodes within the lateral compartment of the neck is suspected, modified radical neck dissection is also indicated at the time of initial surgery. With recurrence rates reaching 20.5% at a mean follow-up of 11.3 years, patients with papillary carcinoma are routinely followed postoperatively by ultrasound (US) examination of both the central and lateral compartments of the neck in conjunction with serum thyroglobulin testing.35 Factors predictive of recurrence include diagnosis at a young age, increased size of the primary neoplasm, extracapsular spread, and the presence of distant metastatic disease most commonly in the lungs or bone.36,37
Recurrent disease is most likely found within the surgical bed or lymph nodes of the central or lateral compartments.38 Traditionally, these patients with recurrent well-differentiated thyroid carcinoma identified on US are taken back to the operating room for reexploration and resection of affected tissue or lymph nodes. However, in patients who have undergone a prior neck dissection, normal tissue planes in the central and lateral compartments are distorted due to scar tissue formation, and reoperative surgery is associated with higher complication rates.39 Recent work at our institution demonstrates that percutaneous RFA may be an effective and relatively safe alternative to surgical management.39,40
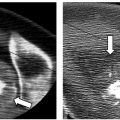
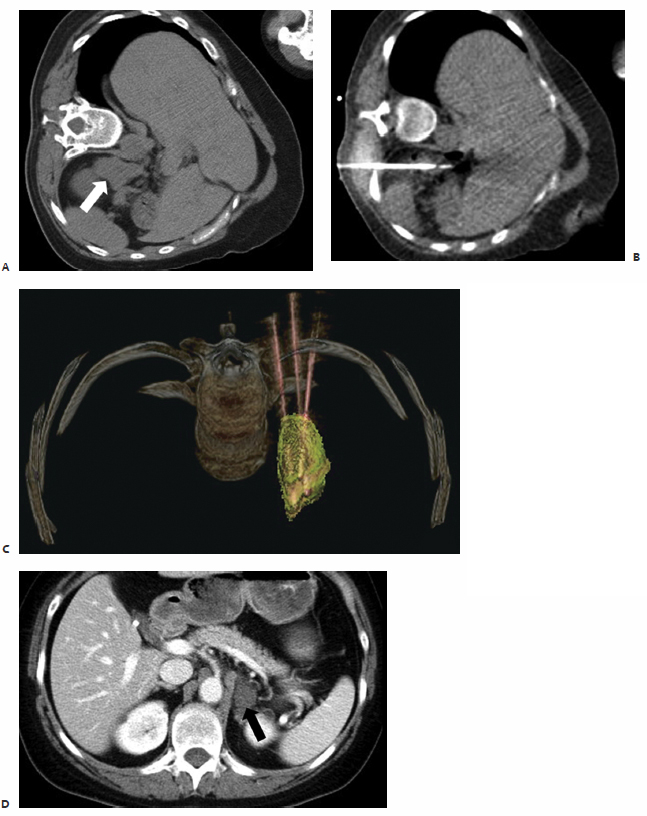
In the first reported study documenting the use of RFA in patients with localized recurrent well-differentiated thyroid carcinoma, Dupuy et al40 demonstrated the percutaneous use of RFA in the treatment of eight patients with biopsy proven recurrence within the neck ( Fig. 9.3 ). Six patients presented with recurrence in the lateral compartment and two with disease in the central compartment. Lesions averaged 2.4 cm in size, ranging from 0.8 to 4.0 cm, with a mean tumor volume of 3.7 mL. Subsequent work by Monchik et al39 reported on an expanded cohort of such patients, bringing the total to 16. As detected by US examination, three patients presented with a recurrent mass within the surgical bed, and the majority, 13 patients, presented with lymph nodes in the lateral neck found to be positive for recurrence on US-guided fineneedle aspiration (FNA) biopsy.39
Twelve of these 13 patients had undergone a modified radical neck dissection at the time of initial surgery. Dupuy et al40 recommend that RFA be used to treat patients with a local recurrence in the lateral compartment only after the patient has undergone a modified radical neck dissection. If a positive node is found within a previously unexplored lateral compartment, chances are there is more than a single affected node, and surgical intervention is appropriate prior to ablation. Exceptions may be made if only one or two sites are identified, both of which are amenable to percutaneous ablation. Pathology was consistent with papillary carcinoma in 15 patients, with one reported case of medullary thyroid carcinoma. A paratracheal lymph node and nodes in close association with the right common carotid artery were treated as well as nodes within the right submandibular region, jugular chain, retroauricular space and bilateral supraclavicular regions, thyroid beds, and lateral neck compartments.40
Anatomic Considerations
The thyroid gland is anatomically situated within the anterior aspect of the lower neck and consists of two lateral lobes extending from the fifth cervical to the first thoracic vertebra connected medially by the isthmus. Arterial blood is supplied by the superior and inferior thyroid arteries and venous drainage by the superior, middle, and inferior thyroid veins. In close association with the inferior thyroid artery is the recurrent laryngeal nerve, a branch of the vagus nerve that provides motor and sensory innervation to the larynx. The left and right recurrent laryngeal nerves descend into the thorax, with the left looping under the aortic arch and the right under the subclavian artery, before coursing cranially within the tracheoesophageal groove to innervate the laryngeal muscles of the neck. Positioned on both sides of the trachea, and immediately posterior to the thyroid gland, the recurrent laryngeal nerve is vulnerable to mechanical injury during surgical dissection as well as thermal injury during RFA.
The treating radiologist should exercise extreme caution when ablating lesions in the lateral aspect of the central compartment due to the close proximity of the recurrent laryngeal nerve. As a precautionary measure against thermal nerve injury, hydrodissection may be used in a manner similar to that described previously for adrenal ablation. Under image guidance, a solution of 5% dextrose in water may be injected between the target lesion and expected anatomic location of the nerve acting to physically displace the nerve and create a protective thermal barrier from the delivered RF energy.39 Thermally induced damage to blood vessels is not a concern due to the well-described vessel-mediated cooling or “heat-sink” effect associated with RFA.
Technique
All patients scheduled to undergo ablation at our institution are routinely seen in outpatient consultation, and the standard departmental RFA protocol is followed in the treatment of all patients who undergo ablation of local recurrent well-differentiated thyroid cancer. Unique to this patient population, as documented in the studies by Dupuy et al40 and Monchik et al,39 preablation workup includes US-guided FNA biopsy in all patients for pathologic confirmation of recurrent disease. Intraprocedurally, grounding pads are placed on all patients in accordance with the manufacturer’s specifications. Conscious sedation is administered by dedicated nursing professionals, who continuously monitor ECG tracings and vital signs.
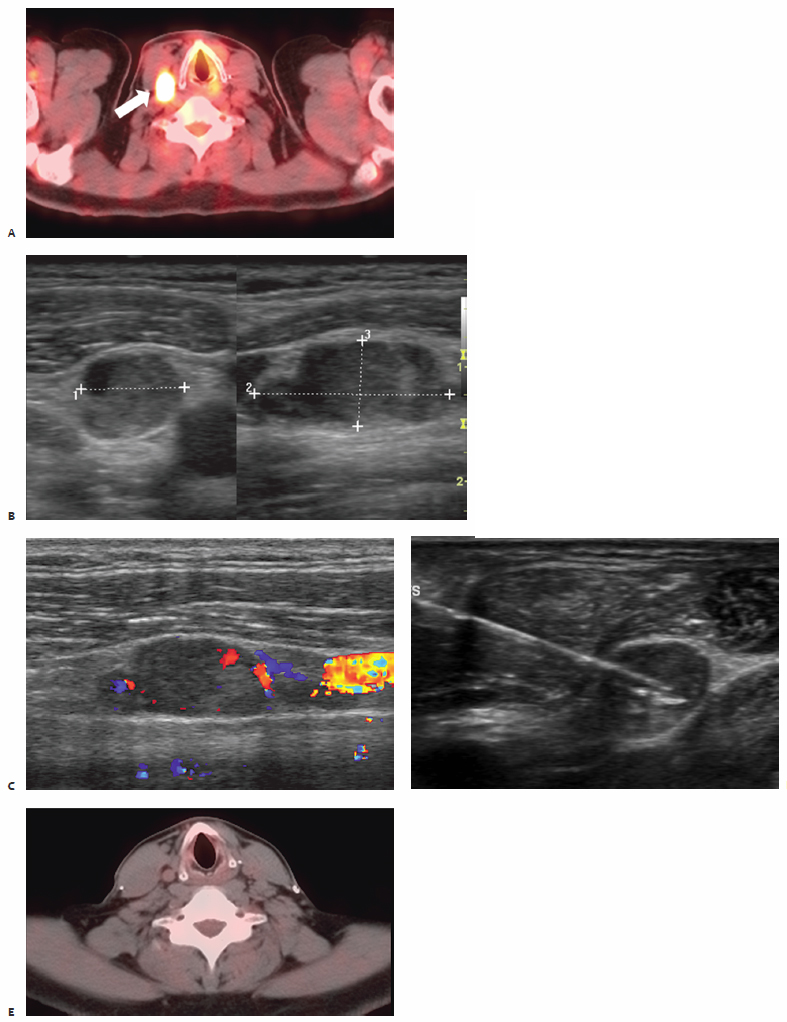
All procedures are performed under real-time US guidance (10 to 14 MHz linear US transducer) enabling proper intralesional placement of a single internally cooled RF electrode with an active-tip length of 1 or 2 cm (Cool-Tip; Valley Laboratory/Covidien, Boulder, CO) powered by an RF generator (Cosman Coagulator-1, Valley Laboratory/Covidien). Target lesions are treated with the maximum allowable current for 2 to 12 minutes, and cytotoxic temperatures are maintained at 50° to 90°C for a minimum of 2 minutes to ensure adequate induction of cytotoxic conditions. Multiple RF applications are performed per session at the discretion of the treating radiologist. The largest lesion in the Dupuy et al40 series measured 4 cm and required five individual applications. Postablation electrode temperature readings >50°C as well as real-time intraprocedural observation of micro-bubble formation and loss of intralesional vascular flow on color-Doppler images served as session end points indicative of appropriate thermocoagulation.
Postablation Management, Efficacy, and Follow-Up
All patients in studies by both Dupuy et al40 and Monchik et al39 who underwent percutaneous RFA of local recurrent well-differentiated thyroid carcinoma in either central or lateral neck compartments were monitored in the postprocedure recovery area until all sedative effects resolved. Patients were discharged in accordance with the department’s protocol within 2 to 6 hours. Postprocedural discomfort was treated with acetaminophen or hydrocodone/acetaminophen. Routine follow-up included a 1- to 2-week postprocedural visit and thereafter regularly scheduled outpatient visits to the tumor ablation clinic. Patients were monitored by means of physical examination, serum thyroglobulin laboratory values (calcitonin levels in the case of medullary carcinoma), and high-resolution ultrasonography at 6-month intervals.
In the initial work documented by Dupuy et al,40 all eight patients tolerated thermal ablation to completion; several of the patients underwent treatment at multiple sites. One patient was found on follow-up examination to have suspicious findings, prompting biopsy, and the patient underwent reablation at that site. A second patient, who had undergone RFA of two individual recurrences at the time of initial ablation, was found at 2-year follow-up to have a recurrence at one of the ablated sites, which necessitated retreatment. One of the eight patients died of advanced systemic metastatic disease 10 months following ablation, and the remaining seven demonstrated no uptake in the neck on follow-up total body scans.40
A reduction in thyroglobulin levels was observed in six of the eight patients, and US examination at 3 months post-ablation showed loss of hypervascularity on color Doppler, which was present in seven patients prior to treatment. Following thermal ablation, ablated lymph nodes were no longer hypoechoic but rather echogenic relative to muscle, with a mean reduction in size from 2.4 to 1.8 cm and continued shrinkage at the 6-month follow-up.40 It is advised that any lymph nodes that are found in postablation imaging studies to have increased in size may harbor residual tumor, and they require US-guided FNA biopsy.
Additional data subsequently presented by Monchik et al39 showed no evidence of recurrent disease at the site of RFA in 14 of the 16 treated patients at a mean follow-up period of 40.7 months. All 16 patients reported self-limited regional discomfort and neck swelling lasting only 1 to 2 weeks following ablation. No infectious complications were reported, nor burns at the sites of grounding pad placement. Procedure-related complications did, however, include a minor skin burn. The burn measured 0.5 cm in diameter and was located at the percutaneous RF electrode entry site due to contact of the proximal portion of the electrode active tip with the patient’s skin. A second patient who underwent RFA of recurrent papillary carcinoma in the central compartment, subsequent to total thyroidectomy, suffered permanent vocal cord paralysis due to thermal injury of the recurrent laryngeal nerve. This presented as audible hoarseness immediately following ablation, and despite symptomatic improvement over the following 2 months, laryngoscopic examination confirmed right vocal cord palsy.39
Further efficacy of RFA of local recurrent well-differentiated thyroid carcinoma is demonstrated by the work of Solbiati et al,41 who report on a series of two patients with papillary carcinoma metastatic to supraclavicular lymph nodes. Lesions ranging in size from 1.5 to 2.0 cm were ablated under US guidance using internally cooled electrodes, and complete necrosis without evidence of recurrent disease was found at ablation sites in both patients at 1-year follow-up.41 Thus, percutaneous RFA has emerged as a viable minimally invasive alternative to surgical management and high-risk reexploration in patients who have already undergone thyroidectomy (partial or complete), radioactive iodine ablation, and lymph node dissections. Ablated sites are amenable to future imaging and US-guided biopsy if indicated in longitudinal follow-up, and lesions <0.1 cm are identifiable. Unlike radioactive iodine, RFA may be repeated in an area of prior treatment as deemed necessary by the treating radiologist.
♦ Head and Neck Tumors
Patient and Tumor Selection
Worldwide incidence of head and neck cancers is reported to exceed 500,000 cases each year, with an annual incidence of 40,490 cases in the United States in 2006, accounting for approximately 3% of adult malignancies. An associated mortality of 11,170 was also reported in the U.S. that same year.42 Substantial morbidity is reported in these patients, who commonly suffer from intractable pain, cranial neuropathies, otalgia, odynophagia, dysphagia, trismus, and limited tongue mobility, making oral consumption of daily caloric requirements a challenge.43 It is estimated that two thirds of patients present with locally or regionally advanced disease, and with conventional treatment options, including surgery and adjuvant radiation therapy, 5-year survival rates average 55%.44,45 As speech and swallow mechanisms and functionality decline secondary to chemotherapy, intraarterial chemoinfusion, and radiation, patients commonly refuse additional necessary treatment. Compounding this are the poor rates of local control and survival as well as minimal improvement in quality of life. Additionally, only a small percentage of patients are surgical candidates due to extensive local disease, involvement of vital structures, and medical comorbidities. Thus, clinical studies propose that percutaneous RFA, a safe and effective minimally invasive treatment modality, may improve the quality of life in patients with head and neck cancers.
The work of Brook et al43 details the use of CT-guided RFA in the palliative treatment of 14 patients with recurrent advanced head and neck malignancies. All patients had previously undergone surgery, radiation, and chemotherapy, subsequently presenting with biopsy-proven stage IV disease. Palliation was sought for symptoms including pain, airway obstruction, dysphagia, and poor oral/jaw mobility—all secondary to aggressive tumor growth. Malignancies treated included squamous cell carcinoma of the tongue, tonsil, oropharynx, and maxillary sinus, as well as medullary thyroid carcinoma, basal cell carcinoma of the skull base, and an angiomatoid fibrous histiocytoma of the neck and posterior fossa. Lesion sizes ranged from 3.5 to 10.0 cm, with a mean of 6.3 cm.43
A case report by Bui and Dupuy46 documents the treatment of an adenoid cystic carcinoma of the salivary gland, a rare malignancy associated with poor patient prognosis and an aggressive natural history, with the potential for local extension and distant metastasis. As most lesions are in close proximity to vital neurovascular structures, wide local excision is usually not feasible, making reoperative intervention and radiation necessary due to frequent local recurrences. This symptomatic, recurrent adenoid cystic carcinoma in the right temporal bone extending posteriorly to abut the cerebellum measured 3 × 4 × 4 cm. There was associated destructive involvement of the petrous portion of the right temporal bone and right skull base.46
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree