9 Seizures
9.1 Introduction
Seizures are a common neurologic finding in children, with approximately 400,000 children in the United States having epilepsy. In addition to patients with documented seizures of varying types (Table 9-1), it is common for studies to be requested to evaluate seizure like activity, such as staring spells or pseudoseizures. Although uncomplicated febrile seizures do not require imaging of the brain, nearly all other instances of seizures in children will eventually result in imaging. Accordingly, this is a common indication for imaging in pediatric neuroradiology, and an understanding of the disease processes in seizure disorders, and a logical approach to establishing protocols for imaging and to image interpretation, is critical.
Type | Description | |
Focal | Previously called partial seizures, and previously subdivided into simple partial and complex partial seizures. Seizures that are (on a conceptual basis) believed to originate and remain within a single cerebral hemisphere. | |
Generalized (seizures that involve both cerebral hemispheres, typically with alteration and/or loss of consciousness) | Absence | Brief loss of consciousness without an appreciable postictal state (formerly classified as petit mal seizures) |
Myoclonic | Brief shock-like muscle jerking | |
Tonic | Sudden increased tone of a muscle or group of muscles. Consciousness is usually preserved. | |
Clonic | Alternating contraction and relaxation of a muscle or group of muscles | |
Tonic–clonic | Increased tone within a muscle or group of muscles, followed by alternating contraction and relaxation (formerly designated grand mal seizures). When prolonged (>10 minutes), this is referred to as status epilepticus, which is a medical emergency. | |
Atonic | Sudden loss of muscle tone, often resulting in the patient falling (“drop attack”) | |
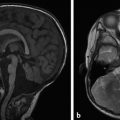
Gelastic | Seizures that may manifest as laughing spells, typically associated with a hypothalamic hamartoma | |
Used with permission from Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51(4):676–85. | ||
9.2 Imaging Modalities
In the emergent setting, computed tomography (CT) may be done on a patient with a seizure so as to rapidly exclude intracranial hemorrhage or other acute abnormalities (particularly post traumatic seizures). However, magnetic resonance imaging (MRI) is predominantly the mainstay modality for the evaluation of seizures. In the absence of signs of infection or known predisposing factors (e.g., a neurocutaneous disorder), or signs of a tumor (in particular ganglioglioma and dysembryoplastic neuroepithelial tumor), intravenous contrast enhancement is typically not required for the evaluation of seizures through MRI. Vascular imaging, such as computed tomographic angiography (CTA) or magnetic resonance angiography (MRA), may help if there are signs of vascular abnormality or prior stroke.
Certain surgical procedures can aid in the treatment of some patients with epilepsy (Table 9-2). In patients who are considered candidates for epilepsy surgery, advanced imaging modalities, including functional MRI (fMRI), and in nuclear medicine, single-photon emission computed tomography (SPECT) perfusion and positron emission tomography (PET), may be useful; these are discussed in the surgical planning section of this chapter. Magnetoencephalography is a technique that detects the focal magnetic field that arises from neuronal activation and can aid in the spatial localization of epileptic discharges with greater accuracy than can surface electroencephalography (EEG). However, although magnetoencephalography is a helpful technique, it is not widely available at present.
Surgery | Description |
Temporal lobectomy | Surgical resection of the temporal lobe. Temporal lobectomy procedures are often characterized by surgeons as the distance from the temporal pole that was resected (e.g., a 3-cm temporal lobectomy vs. a 4-cm temporal lobectomy). This usually involves resection of the temporal pole, uncus, and amygdala, and may or may not involve resection of the head of the hippocampus. |
Corpus callosotomy | Transection of the corpus callosum, often to prevent seizure propagation in patients with atonic seizures, so as to prevent drop attacks (i.e., the patient retains control of half of the body and does not fall). The callosotomy may involve the entire corpus callosum, spare the splenium (sometimes called a 90% callosotomy), or spare the splenium and isthmus (sometimes called a 70% callosotomy). |
Hemispherectomy (functional) | Resection and/or disconnection of a cerebral hemisphere. This involves a temporal lobectomy, resection of the insula and portions of the frontal and parietal lobes, and a corpus callosotomy to disconnect the remaining parenchyma. |
Topectomy | A focal surgical resection of an epileptogenic focus, possibly involving a structural lesion, such as cortical dysplasia or a cavernoma. |
Vagal-nerve stimulator | An implanted device with an electrode that wraps around the vagal nerve and intermittently stimulates it. |
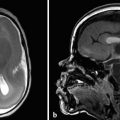
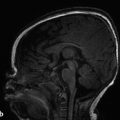
Understanding the normal hippocampal anatomy is critical for the evaluation of seizures because both developmental and acquired abnormalities of the hippocampus can be associated with seizures (Fig. 9.1). A normal variant in the hippocampal region that warrants awareness is a choroidal-fissure cyst; if the adjacent parenchyma is normal, and the cyst contents follow cerebrospinal fluid (CSF) behavior in all sequences, including suppression on fluid-attenuated inversion recovery (FLAIR) facilitated diffusion, as well as lack of enhancement with the administration of contrast medium, then what is being seen is a normal hippocampal variant, usually without pathologic consequence (Fig. 9.2).


9.3 Mesial Temporal Sclerosis/Hippocampal Sclerosis
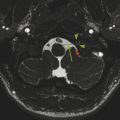
Evaluation of the hippocampi, particularly in the coronal plane, is important in all seizure evaluations. Hippocampal volume loss with a hyperintense signal on T2 W/FLAIR imaging, related to gliosis, is a feature of mesial temporal sclerosis (MTS), also known as hippocampal sclerosis (Fig. 9.3). The entire hippocampus may be involved, although other patterns of involvement, such as of sections CA1 and CA4, or isolated involvement of CA4 (end-fimbrial sclerosis), have been described. It is important to be aware that this process can be bilateral in up to 20% of patients with MTS, which carries two potential diagnostic pitfalls. First, a bilateral symmetric abnormality can be missed unless there is familiarity with the normal hippocampal morphology. Second, a bilateral asymmetric abnormality could be mistaken for a unilateral process, which is important because in some cases the less atrophied side of the hippocampus may be the cause of seizures. Therefore, caution is warranted before the structural findings suggestive of MTS are definitively linked with the physiologic implications of the location of origin of a seizure. Determining the true cause of seizures is the reason for more detailed workup, including EEG, possible intraoperative monitoring with grid electrodes or depth electrodes, SPECT perfusion studies, and possibly MEG. An imaging feature supportive of MTS is volume loss in the ipsilateral fornix and mammillary body, related to Wallerian degeneration along the circuit of Papez.

Mesial temporal sclerosis has been shown to be more common in individuals with a childhood history of febrile seizures, but the exact cause–effect relationship of the two disorders remains unclear. Although MTS is typically a disease of adolescence and adulthood, it can be seen in children as young as 2 years of age who have a mutation of the cellular type 1A voltage-gated sodium channel (SCN1A).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree