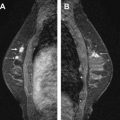
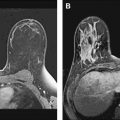
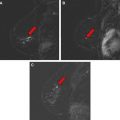
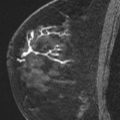
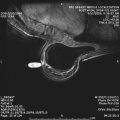
Magnetic resonance (MR) imaging identifies cancer not found by clinical examination or other breast imaging studies, but its effect on patient outcomes is controversial. To date, its use has not been shown to increase the likelihood of obtaining negative surgical margins, decrease the rate of conversion from lumpectomy to mastectomy, or decrease local recurrence. The rate of tumor identification with MR imaging is 2 to 3 times higher than the incidence of local recurrence, resulting in mastectomies that may not be beneficial to the patient. This is also a concern with the use of MR imaging for contralateral cancer detection. The use of MR imaging for early detection of local recurrence does not take into account what is known about the biology of local recurrence because a short interval to local recurrence is associated with poor prognosis. In problem areas, such as evaluation of response to neoadjuvant therapy and detection of cancer presenting as axillary adenopathy, MR imaging provides information that is useful for clinical management.
Breast cancer mortality in the United States, as well as in other parts of the world, has decreased in recent years, a finding attributable in part to the use of screening mammography and in part to improved treatment, particularly the use of adjuvant endocrine therapy and chemotherapy. It is now recognized that breast cancer survival and local control are a function of the interaction between the fundamental biology of the cancer, the disease burden, and the availability of effective therapy. Traditionally, disease burden has been the major consideration in selecting local therapy. In patients treated with breast-conserving therapy (BCT) consisting of excision of the tumor and whole-breast irradiation, randomized trials have shown that failure to reduce the tumor burden in the breast to a subclinical level is associated with an increased risk of local recurrence. This has resulted in multicentric cancer and margins that cannot be cleared of cancer cells being accepted as indications for mastectomy. As experience with BCT has been gained, local recurrence rates have steadily decreased as a result of the routine inking of specimen margins, more extensive pathologic evaluation of margin surfaces, standardization of radiation doses, and the widespread use of adjuvant endocrine therapy and chemotherapy.
Magnetic resonance (MR) imaging is a technology capable of detecting small foci of cancer in the breast that were previously only evident on detailed pathology evaluation. Although it seems intuitively obvious that an improved ability to detect cancer would be beneficial, evidence of improved patient outcomes is lacking. This article considers the available data on the effect of MR imaging on short- and long-term outcomes of surgical treatment of operable breast cancer based on what is known today about patient selection for BCT and the incidence, detection, and management of local recurrence. Other relevant clinical areas, including the effect of MR imaging on selection of patients for partial breast irradiation, and its role in the multidisciplinary care of the patient receiving neoadjuvant therapy, are also addressed. When considering therapy from a clinical perspective, oncologic end points of relevance to the patient, such as disease-free survival or quality of life, and not the rate of cancer detection, must be considered. The authors have attempted to address these end points in the context of current thinking regarding the role of local control in improving survival and what is known about the biologic diversity of breast cancer.
MR imaging for treatment selection
Clinically and radiographically, breast cancer is usually a unicentric lesion, with multicentric carcinoma identified by physical examination or mammography in fewer than 10% of cases. However, pathology studies using serial subgross sectioning of mastectomy specimens have documented that additional tumor foci are frequently present in the same quadrant (multifocal) or other quadrants (multicentric) of a breast with what seems to be clinically unicentric tumor.
In one such study, Holland and colleagues evaluated mastectomy specimens of 282 patients with unicentric cancers 5 cm or less in size and found additional tumor foci in 63%. In 20% of cases, the additional tumor was identified within 2 cm of the index tumor, and in the remaining 43% at a greater distance from the primary site, although usually within 4 cm. The likelihood of identifying additional tumor foci was not related to the size of the primary tumor.
Studies examining the incidence of pathologic multifocality or multicentricity in clinically localized tumors are summarized in Table 1 . In spite of variable inclusion criteria, additional tumor foci were identified in 21% to 63% of cases, including a 44% incidence in patients with mammographically detected lesions. Such studies were initially used to argue that the treatment of breast cancer with approaches that did not remove the entire breast was inappropriate and would result in high rates of local recurrence. Extensive clinical experience, including multiple prospective randomized trials, has since demonstrated that survival after BCT, consisting of excision of the tumor to negative margins and whole-breast irradiation, is equal to survival after mastectomy. In the current era of the routine use of adjuvant systemic therapy in breast cancer, 10-year rates of local recurrence after BCT are less than 10%, considerably lower than the incidence of multifocality/multicentricity seen in the pathology studies included in Table 1 . These findings indicate that although subclinical tumor foci are present in significant numbers of women with clinically localized breast cancer, most of these subclinical tumor foci are controlled with radiotherapy (XRT), and it is this paradox that lies at the heart of the debate over the benefit of MR imaging for cancer staging and treatment selection.
| Study | No. of Cases | Population | Multifocal/Multicentric (%) |
|---|---|---|---|
| Qualheim and Gall | 157 | Not stated | 54 |
| Rosen et al | 203 | Invasive carcinoma | 33 |
| Lagios | 85 | Not stated | 21 |
| Egan | 118 | Not stated | 60 |
| Schwartz et al | 43 | Nonpalpable cancer | 44 |
| Anastassiades et al | 366 | Invasive ≤7 cm, noninvasive | 49 |
| Holland et al | 282 | Clinically unicentric invasive cancer <5 cm | 63 |
It is clear that, in women with breast cancer, MR imaging identifies additional cancer foci that are not evident on clinical examination, mammogram, or ultrasound. In a meta-analysis of 19 studies, including 2610 patients with breast cancer, Houssami and colleagues reported that additional cancer was identified by MR imaging in 16% of patients, with a range of 6% to 34% in individual studies. In studies restricted to patients with infiltrating lobular carcinoma, the detection of additional disease is even more frequent.
In a meta-analysis of MR imaging in lobular carcinoma reported by Mann and colleagues, which included 18 studies and 450 cancers, additional disease was detected with MR imaging in 32% of cases (95% confidence interval [CI] 22%–44%). The usefulness of MR imaging in ductal carcinoma in situ (DCIS) is unclear.
Some studies have reported that MR imaging is less effective at detecting DCIS than invasive carcinoma. Sardanelli and colleagues observed a sensitivity of only 40% for the detection of DCIS by MR imaging when the results of serial subgross sectioning were used as the standard. In contrast, Kuhl and colleagues reported that MR imaging was significantly more sensitive than mammography for the detection of DCIS when conventional pathologic evaluation was used. Of 167 women with DCIS who had undergone mammography and MR imaging preoperatively, DCIS was diagnosed by mammography in 56% of cases, and by MR imaging in 98%, with the superior performance of MR imaging particularly evident in high-grade DCIS.
Although it is possible that some of the enhancement patterns seen with DCIS were not well recognized as abnormal in early studies of MR imaging, resulting in a lower sensitivity, the clinical questions regarding the significance of the low-volume disease detected by MR imaging, whether DCIS or invasive carcinoma, are similar.
It is reasonable to ask whether the tumor foci identified by MR imaging are the same tumor foci identified by pathologists using serial subgross sectioning. This question was most directly addressed by Sardanelli and colleagues, who performed MR imaging on 90 patients before mastectomy, then processed the mastectomy specimens with serial subgross sectioning and correlated the pathologic tumor location with the findings of the preoperative MR imaging. The overall sensitivity of MR imaging for the detection of tumor was 81%; 89% for invasive carcinoma and 40% for DCIS. In the 90 breasts studied, MR imaging failed to identify microscopic multifocal or multicentric disease in 19, and incorrectly indicated additional disease in 30, and correctly identifying the extent of tumor in 50. The mean diameter of malignant lesions not seen by MR imaging was 5 mm and ranged from 0.5 mm to 15 mm. These findings strongly suggest that MR imaging is capable of finding some, but not all, of the tumor foci identified with detailed pathologic sectioning.
Indirect evidence also suggests that the same tumor is identified with both techniques. Berg and colleagues observed that in 40 of 46 breasts with additional tumor foci detected with MR imaging, the tumor foci were within 4 cm of the index lesion. Liberman and colleagues also noted that most of the additional tumors detected were in the same quadrant as the index lesion. These findings correspond well to the observations of Holland and colleagues that 96% of pathologically detected tumor foci were within 4 cm of the index tumor, and provide further support for the concept that MR imaging is a technology capable of detecting some, but not all, of the tumors seen on serial subgross sectioning.
Until relatively recently, it has been assumed that the finding of additional cancer on MR imaging was clearly of benefit to the patient. In the meta-analyses of Houssami and colleagues, the results of the MR imaging examination changed surgical therapy in 7.8% to 33.3% of cases in individual studies, and virtually always in the direction of more extensive surgery, such as a wider excision or a mastectomy that would not otherwise have been performed. In a study of 5405 patients treated at the Mayo Clinic, Rochester, MN, the use of MR imaging increased from 10% of newly diagnosed breast cancers in 2003 to 26% in 2006. A significant increase in the mastectomy rate was also observed during this period, and after adjustment for age, stage, and the presence of contralateral carcinoma, women who had MR imaging were 1.7 times more likely to undergo mastectomy than their counterparts who did not have the examination, although the mastectomy rate was also noted to increase in this group over time. If the more extensive surgery that occurs as a result of MR imaging findings is truly beneficial to patients, it should result in improvement in either short-term outcomes of surgery, such as the improved ability to identify patients requiring a mastectomy preoperatively, or an increased likelihood of achieving negative margins with a single operative procedure. Alternatively, the benefit of MR imaging may be to improve long-term outcomes by decreasing the incidence of local recurrence after BCT or allowing the synchronous detection of contralateral breast cancer.
The effect of MR imaging on short-term surgical outcomes
The identification of patients who are appropriate candidates for BCT is not a major problem at the present time. Standard guidelines for the use of BCT have been developed, and contraindications to the procedure are reliably identified with a history, physical examination, and diagnostic mammography. Morrow and colleagues reported that of 263 consecutive patients selected for BCT between 1989 and 1993 using a history, physical examination, and diagnostic mammography, conversion to mastectomy was necessary in only 2.9%. In a population-based sample of 800 women from the Los Angeles and Detroit Surveillance Epidemiology and End Results (SEER) sites attempting BCT between June 2005 and May 2006, 12% required conversion to mastectomy, although in 8%, mastectomy occurred after a single attempt at BCT. Two retrospective studies and 1 prospective randomized trial have examined whether the use of MR imaging reduces the need for mastectomy in patients attempting BCT. Bleicher and colleagues retrospectively reviewed 290 patients attempting BCT who had a multidisciplinary preoperative evaluation between July 2004 and December 2006, and found no significant difference in the likelihood of requiring conversion from BCT to mastectomy based on a preoperative MR imaging. Pengel and colleagues compared outcomes among 355 women treated at a single institution. Those who had an MR imaging were part of a study evaluating the procedure, and the control group consisted of patients who declined to enter the study. Again, no significant differences in the rate of unanticipated conversion to mastectomy were noted ( Table 2 ).
| Author | No. of Patients | Mastectomy (%) | P Value | |
|---|---|---|---|---|
| No MR Imaging | MR Imaging | |||
| Bleicher et al | 290 | 5.9 | 9.8 | NS |
| Pengel et al | 355 | 5.1 | 2.5 | NS |
| Turnbull et al | 1623 | 7.6 | 5.9 | NS |
The most robust data addressing this question came from the COMICE trial, a prospective randomized study involving 107 participating surgeons in the United Kingdom that was designed to address the question of the role of MR imaging in improving outcomes in patients undergoing BCT. The sample included 1623 women believed to be candidates for BCT who were stratified by age, breast density, and treating surgeon, and randomized to receive an MR imaging scan or not. In the MR imaging group, 58 patients (7.1%) underwent an immediate mastectomy as a result of the MR imaging findings, and 10 patients in the no MR imaging group (1.2%) changed their treatment decision after randomization and opted for a mastectomy. Of the remainder who attempted BCT, conversion to mastectomy was required in 5.9% of the MR imaging group and 7.6% of the no MR imaging group ( P = NS, see Table 2 ). The overall result was a 13% mastectomy rate in the MR imaging group and an 8.8% rate in the no MR imaging group. In the study of Pengel and colleagues, the mastectomy rate was doubled in the MR imaging group (11.6% vs 5.1%), and in the report of Bleicher and colleagues, the use of MR imaging increased the mastectomy rate from 25% to 38%, an odds ratio of 1.80 after adjustment for tumor size and patient age ( P = .024). These studies provide no suggestion that MR imaging decreases the likelihood of unplanned mastectomy, but do show a consistent pattern of an increased mastectomy rate in patients undergoing MR imaging.
In contrast to patient selection for BCT, which is not a major clinical problem, the need for reexcision because of margins involved with tumor after the initial lumpectomy is a common clinical occurrence. Morrow and colleagues observed that 22% of 800 women undergoing successful BCT in a population-based sample derived from the SEER registry required at least 1 reexcision to complete surgical therapy. Reexcision is traumatic to patients, costly to the health care system, and delays the initiation of adjuvant therapy. Strategies to reduce the need for reexcision would address a substantial problem in breast cancer surgery. Table 3 summarizes 4 retrospective studies and 1 prospective study that have addressed the question of the effect of MR imaging on the need for reexcision. In spite of the inclusion of almost 2500 patients in total, none of the individual studies show a significant reduction in the need for reexcision in patients undergoing MR imaging. One study limited to patients with infiltrating lobular carcinoma has shown a benefit for MR imaging. Mann and colleagues studied 90 patients who did not have MR imaging and 55 who did in whom BCT was attempted and reported a 27% rate of reexcision in the no MR imaging group compared with 9% in the MR imaging group ( P = .01), as well as a significantly higher mastectomy rate in the no MR imaging group (23% vs 7%; P = .013). Patients included in this study were treated between 1993 and 2005, and it is not clear if those undergoing MR imaging were more commonly treated later in the study period and if criteria for reexcision were uniform over time. Other studies have not confirmed a difference in the need for reexcision in patients with infiltrating lobular and infiltrating ductal histology. In a study of 318 patients with infiltrating lobular cancer who were matched by stage, year of diagnosis, and menopausal status to 2 controls with infiltrating ductal cancer (n = 636), 25% of patients with lobular cancer required reexcision compared with 21% of those with ductal cancer, a difference that was not statistically significant after adjusting for tumor size and patient age. One potential explanation for the inability of MR imaging to reduce the need for reexcision has to do with its accuracy in determining tumor size. Although multiple studies have shown that MR imaging is more accurate than mammography in determining tumor size, the degree of precision of measurement, and the ability to translate the imaging findings to the amount of tissue removed in the operating room, may be insufficient to see a reduction in margin positivity. For example, Grimsby and colleagues compared tumor size as determined by MR imaging for 190 invasive breast cancers with pathologic size measurements and found that MR imaging estimated size within 5 mm of the pathologic measurement in only about half of cases, overestimating the size in one-third of cases, and underestimating in 15%. As previously discussed, when serial subgross sectioning was used as the standard, MR imaging underestimated disease extent for 19% of invasive foci and a far higher percentage of DCIS foci. Because margin specimens are often subjected to detailed pathologic scrutiny, some of this disease will be identified as positive margins.
| Author | No. of Patients | % Positive Margins | P Value | |
|---|---|---|---|---|
| No MR Imaging | MR Imaging | |||
| Bleicher et al | 290 | 14 | 22 | NS |
| Pengel et al | 355 | 19 | 14 | NS |
| Schiller et al | 730 | 18 | 14 | NS |
| Hwang et al | 472 | 14 | 12 | NS |
| Turnbull et al | 1623 | 11 | 10 | NS |
The available data on the use of MR imaging in the setting of newly diagnosed breast cancer do not provide evidence of patient benefit in short-term surgical outcomes, and raise some concerns. In addition to the increased mastectomy rate seen in patients undergoing MR imaging (discussed earlier), there are also concerns about false-positive findings and the need for additional radiologic workup to evaluate these findings, leading to increased health care costs and delays in therapy. In the meta-analysis of Houssami and colleagues, the false-positive rate of MR imaging was 5.5% (95% CI 3.1%–9.5%), and it is likely that false-positive rates outside of the centers of expertise included in this meta-analysis are higher. Petit and colleagues reported that 36 of 410 patients believed to be candidates for BCT were converted to mastectomy because of additional MR imaging lesions. In 23 cases, biopsy confirmation of malignancy in the additional lesion was not performed, and no additional cancer was found in more than half of these patients. Although it is clear that the problem of inappropriate surgery because of false-positive MR imaging results can be minimized with biopsy confirmation of malignancy, there are some practical difficulties associated with this approach.
The current algorithm for evaluating an MR imaging abnormality involves a targeted ultrasound to try and identify the lesion to allow an ultrasound-guided biopsy. If the lesion cannot be visualized by ultrasound and the patient is seeking treatment at a different institution than the 1 in which the MR imaging was obtained, then the MR imaging is often repeated to verify the presence of a target before the time of biopsy. In the study of Bleicher and colleagues, there was a 22.4-day delay in the time from histologic diagnosis to initial surgery in patients who had MR imaging compared with those who did not ( P = .01). The need for additional biopsies, particularly at multiple sites, is traumatic for patients, and Berg and colleagues found that 12% of patients underwent a medically unnecessary mastectomy rather than undergo further workup of abnormal MR imaging findings. The risk of unnecessary surgery is present in the ipsilateral and the contralateral breast. King and colleagues compared presenting characteristics of 2558 women who underwent unilateral mastectomy with 407 who had a contralateral prophylactic mastectomy (CPM) between 1997 and 2005 at Memorial Sloan-Kettering Cancer Center. Patients having preoperative MR imaging were significantly more likely to undergo CPM (43% vs 16%; P = .0001), and this was particularly true if the unaffected breast required a biopsy for a benign finding.
Effect of MR imaging on long-term cancer outcomes
Local Recurrence
A potential major benefit to patients of preoperative MR imaging would be a reduction in the incidence of local recurrence after BCT. Since the publication of the initial trials that established the suitability of BCT as a breast cancer treatment modality, rates of local recurrence have steadily declined. This decrease can be attributed to improvements in mammography, the routine inking of surgical margins and more detailed evaluation of margin specimens, and particularly to the increased use of adjuvant systemic therapy. Pass and colleagues examined the effect of changes in the processes of care between 1981 and 1996 on local recurrence rates in a group of 607 patients treated at a single institution. Between 1981 and 1985, the 5-year rate of ipsilateral breast tumor recurrence (IBTR) was 8%, decreasing to 1% between 1991 and 1996. In this period, the proportion of patients with negative margins increased from 48% to 76%, and the mean number of pathology slides examined to determine margin status increased from 11 to 21 per patient. The use of tamoxifen increased from 10% to 61% of cases. In a similar study, Ernst and colleagues observed 8-year rates of locoregional recurrence after BCT to decrease from 20.1% between 1985 and 1992, to 5.4% from 1993 to 1999. In contrast, rates of locoregional recurrence after mastectomy did not change between the 2 time periods. In the National Surgical Adjuvant Breast and Bowel Project (NSABP) trials conducted since the 1990s, rates of IBTR at 10 years were less than 8% in node-positive and node-negative women receiving systemic therapy. These findings emphasize that local recurrence may be caused by 2 mechanisms:
The first mechanism, an excessive tumor burden in the breast that cannot be controlled with XRT, is the type of local recurrence that is potentially amenable to reduction through the use of MR imaging for patient selection.
The second mechanism, local recurrence that occurs because of biologically aggressive disease, is actually a first site of metastases and will only be affected by improvements in systemic therapy.
The proportion of local recurrences caused by each of these mechanisms is unknown; however, the observation from the Early Breast Cancer Trialists overview analysis that local recurrence is seen on the chest wall after mastectomy and XRT in 3% of node-negative cases and 7% of node-positive cases (figures similar to current rates of IBTR after BCT) strongly suggests that most recurrences after BCT in the current era are caused by aggressive biology, not a heavy residual disease burden in the breast.
Three studies have retrospectively examined the effect of patient selection with MR imaging on IBTR. Fischer and colleagues retrospectively compared 121 patients who had preoperative MR imaging to 225 who did not. After a mean follow-up of approximately 40 months, IBTR occurred in 1.2% of the MR imaging group and 6.8% of the no MR imaging group ( P <.001). The 6.8% incidence of IBTR at less than 5 years follow-up is unusually high by current standards, making the outcome of this study difficult to interpret. In addition, although patients in the MR imaging group were more likely to have T1 tumors (64% vs 48%), more likely to be node-negative (61% vs 54%), and less likely to have high-grade lesions (13% vs 28%), no adjustments for differences in tumor characteristics between the groups were made. In spite of the more favorable profile of the MR imaging patients, chemotherapy was administered to 95% of patients in this group with indications for treatment, compared with 82% in the no MR imaging group, and no adjustment was made for this difference. The combination of an unusually high rate of IBTR in the no MR imaging group compared with other large datasets of patients treated without MR imaging in the same time period, and the lack of adjustment for major differences in tumor and treatment variables which affect the incidence of IBTR, make it difficult to draw reliable conclusions from this study. Solin and colleagues also examined the effect of MR imaging on IBTR in 215 patients who had the examination and 541 who did not. Appropriate statistical adjustments were made for differences between patient groups. At 8 years, the rate of IBTR in the MR imaging group was 3% and was 4% in the non-IBTR group. Hwang and colleagues also examined the effect of MR imaging on IBTR with adjustment for differences between groups. After a median follow-up of 54 months, the 8-year actuarial rates of local recurrence were 1.8% in the MR imaging group and 2.5% in the no MR imaging group, a nonsignificant difference.
What is noteworthy about this study and the study of Solin and colleagues is that based on the results of the Houssami and colleagues meta-analysis showing a 6% to 11% conversion rate from BCT to mastectomy based on the findings of MR imaging, between 21 and 38 of the patients in the study by Hwang and colleagues and 32 to 60 patients in the study by Solin and colleagues who were treated without MR imaging had inappropriate BCT; yet the actual number of patients who recurred was 9 and 13, respectively. The COMICE trial will provide additional data on MR imaging and IBTR when further follow-up is available. However, the information available now suggests that the use of MR imaging may not have an effect on breast cancer-specific survival.
The Early Breast Cancer Trialists overview showed that, to observe a survival difference at 15 years, differences in local failure rates of 10% or greater between treatments must be present at 5 years of follow-up. Differences of this magnitude are seen in patients treated with BCT with or without XRT, whether node-positive or node-negative, and in node-positive women undergoing mastectomy treated with and without XRT. The rate of IBTR after BCT in patients selected for the procedure without MR imaging is less than 10% at 10 years, so a difference of the magnitude needed to show a survival gain cannot be anticipated with the addition of MR imaging. Even if the group that seems to be at the highest risk for IBTR after BCT (women with estrogen receptor [ER], progesterone receptor [PR], and human epidermal growth factor receptor 2 [HER2]-negative disease ) were to be studied, it is unlikely that MR imaging would result in a survival benefit because these patients also have the highest risk for local recurrence after mastectomy, strongly suggesting that these recurrences are a reflection of aggressive tumor biology rather than a heavy tumor burden in the breast.
Contralateral Cancer
The other long-term outcome with the potential to be affected by MR imaging is the synchronous versus metachronous diagnosis of contralateral breast cancer. Women with unilateral breast carcinoma are recognized as being at increased risk for the development of second cancers, but the absolute magnitude of this risk is relatively low in women who do not have BRCA gene mutations. In 134,501 women diagnosed with unilateral DCIS, stage 1 and stage 2 breast carcinoma between 1973 and 1996 and reported to SEER, the 10-year actuarial risk of a contralateral cancer was 6.1%, and the 20-year risk was 12%. For those less than 45 years of age at initial diagnosis, these figures were 3.1% and 6.2%, respectively. A diagnosis of DCIS was associated with a 6.0% risk of a second cancer at 10 years, and a diagnosis of infiltrating lobular carcinoma was associated with a 6.4% risk at 10 years. Based on these low incidence rates, it is difficult to argue that more intensive surveillance of the contralateral breast added to an annual mammogram is a cost-effective strategy for the general population of women with breast cancer. However, Lehman and colleagues examined the role of MR imaging for evaluation of the contralateral breast in 969 women with unilateral breast cancer. Cancer was detected by MR imaging in 30 women (3.1%) with clinically and mammographically normal breasts within 12 months of the initial breast cancer diagnosis. The mean patient age was 53.3 years, and only 19.6% had 1 or more first-degree relatives with breast cancer. Of the cancers detected, 18 were invasive carcinoma and 12 were DCIS. An additional 3 cases of DCIS not detected by MR imaging were identified in prophylactic mastectomy specimens. The investigators concluded that MR imaging of the contralateral breast at the time of a unilateral breast cancer diagnosis was useful to allow the detection of early-stage carcinoma, and synchronous rather than metachronous treatment of second primary tumors. The same arguments have been used to support the use of mirror image, contralateral breast biopsy, a procedure with similar results. Cody identified contralateral cancer with mirror image biopsy in 3% of 871 women with unilateral cancer and a normal examination and mammogram treated between 1979 and 1993, and half of the cancers were invasive; Pressman reported a 6.2% identification rate with contralateral biopsy in an earlier time period. To reconcile the findings of Lehman and colleagues and the contralateral biopsy studies with the low rates of cancer observed at 5 and 10 years in the SEER study of Gao and colleagues, one must make the assumption that virtually all contralateral cancer that occurs in the first 5 years after diagnosis is present at the time of diagnosis, and that it is all detectable by MR imaging. This seems unlikely and also ignores clinical data that indicate that the use of endocrine therapy reduces the clinical incidence of contralateral breast cancer by 50%. Even the use of conventional chemotherapy reduces contralateral cancer by 20%, raising the distinct possibility that MR imaging of the contralateral breast identifies some cancers that would never become clinically evident, resulting in unnecessary treatment. In a meta-analysis of MR imaging of the contralateral breast, Brennan and colleagues reported a 9.3% incidence of abnormalities detected by MR imaging in the contralateral breast (true-positive plus false-positive), with a positive predictive value (PPV) of 47.9%. In the already anxious woman with a new diagnosis of breast cancer, this relatively low PPV may have significant clinical consequences. In a large study examining factors associated with CPM, King and colleagues found that undergoing a preoperative MR imaging increased the risk of contralateral prophylactic mastectomy by a factor of 3.2 in multivariate analysis. Similarly, Sorbero and colleagues examined the use of CPM in 3606 stage 1 to 3 patients with breast cancer between 1998 and 2005 and found that in multivariate analysis, the use of preoperative MR imaging was associated with an increased use of contralateral prophylactic mastectomy (odds ratio 2.04; P = .001) in women with stage 1 and 2 disease, although the overall rates of contralateral prophylactic mastectomy were significantly lower than those reported by King and colleagues.
In addition, the 1-year follow-up in the study of Lehman and colleagues is insufficient to judge the effect of MR imaging on the incidence of contralateral cancer, and limited clinical data are available that address this question. Solin and colleagues reported a 6% incidence of cancer at 8 years of follow-up in women who did and did not have preoperative MR imaging, indicating that more data are needed before concluding that MR imaging is routinely indicated in women with unilateral cancer for the purpose of screening the contralateral breast.
The effect of MR imaging on short-term surgical outcomes
The identification of patients who are appropriate candidates for BCT is not a major problem at the present time. Standard guidelines for the use of BCT have been developed, and contraindications to the procedure are reliably identified with a history, physical examination, and diagnostic mammography. Morrow and colleagues reported that of 263 consecutive patients selected for BCT between 1989 and 1993 using a history, physical examination, and diagnostic mammography, conversion to mastectomy was necessary in only 2.9%. In a population-based sample of 800 women from the Los Angeles and Detroit Surveillance Epidemiology and End Results (SEER) sites attempting BCT between June 2005 and May 2006, 12% required conversion to mastectomy, although in 8%, mastectomy occurred after a single attempt at BCT. Two retrospective studies and 1 prospective randomized trial have examined whether the use of MR imaging reduces the need for mastectomy in patients attempting BCT. Bleicher and colleagues retrospectively reviewed 290 patients attempting BCT who had a multidisciplinary preoperative evaluation between July 2004 and December 2006, and found no significant difference in the likelihood of requiring conversion from BCT to mastectomy based on a preoperative MR imaging. Pengel and colleagues compared outcomes among 355 women treated at a single institution. Those who had an MR imaging were part of a study evaluating the procedure, and the control group consisted of patients who declined to enter the study. Again, no significant differences in the rate of unanticipated conversion to mastectomy were noted ( Table 2 ).
| Author | No. of Patients | Mastectomy (%) | P Value | |
|---|---|---|---|---|
| No MR Imaging | MR Imaging | |||
| Bleicher et al | 290 | 5.9 | 9.8 | NS |
| Pengel et al | 355 | 5.1 | 2.5 | NS |
| Turnbull et al | 1623 | 7.6 | 5.9 | NS |
The most robust data addressing this question came from the COMICE trial, a prospective randomized study involving 107 participating surgeons in the United Kingdom that was designed to address the question of the role of MR imaging in improving outcomes in patients undergoing BCT. The sample included 1623 women believed to be candidates for BCT who were stratified by age, breast density, and treating surgeon, and randomized to receive an MR imaging scan or not. In the MR imaging group, 58 patients (7.1%) underwent an immediate mastectomy as a result of the MR imaging findings, and 10 patients in the no MR imaging group (1.2%) changed their treatment decision after randomization and opted for a mastectomy. Of the remainder who attempted BCT, conversion to mastectomy was required in 5.9% of the MR imaging group and 7.6% of the no MR imaging group ( P = NS, see Table 2 ). The overall result was a 13% mastectomy rate in the MR imaging group and an 8.8% rate in the no MR imaging group. In the study of Pengel and colleagues, the mastectomy rate was doubled in the MR imaging group (11.6% vs 5.1%), and in the report of Bleicher and colleagues, the use of MR imaging increased the mastectomy rate from 25% to 38%, an odds ratio of 1.80 after adjustment for tumor size and patient age ( P = .024). These studies provide no suggestion that MR imaging decreases the likelihood of unplanned mastectomy, but do show a consistent pattern of an increased mastectomy rate in patients undergoing MR imaging.
In contrast to patient selection for BCT, which is not a major clinical problem, the need for reexcision because of margins involved with tumor after the initial lumpectomy is a common clinical occurrence. Morrow and colleagues observed that 22% of 800 women undergoing successful BCT in a population-based sample derived from the SEER registry required at least 1 reexcision to complete surgical therapy. Reexcision is traumatic to patients, costly to the health care system, and delays the initiation of adjuvant therapy. Strategies to reduce the need for reexcision would address a substantial problem in breast cancer surgery. Table 3 summarizes 4 retrospective studies and 1 prospective study that have addressed the question of the effect of MR imaging on the need for reexcision. In spite of the inclusion of almost 2500 patients in total, none of the individual studies show a significant reduction in the need for reexcision in patients undergoing MR imaging. One study limited to patients with infiltrating lobular carcinoma has shown a benefit for MR imaging. Mann and colleagues studied 90 patients who did not have MR imaging and 55 who did in whom BCT was attempted and reported a 27% rate of reexcision in the no MR imaging group compared with 9% in the MR imaging group ( P = .01), as well as a significantly higher mastectomy rate in the no MR imaging group (23% vs 7%; P = .013). Patients included in this study were treated between 1993 and 2005, and it is not clear if those undergoing MR imaging were more commonly treated later in the study period and if criteria for reexcision were uniform over time. Other studies have not confirmed a difference in the need for reexcision in patients with infiltrating lobular and infiltrating ductal histology. In a study of 318 patients with infiltrating lobular cancer who were matched by stage, year of diagnosis, and menopausal status to 2 controls with infiltrating ductal cancer (n = 636), 25% of patients with lobular cancer required reexcision compared with 21% of those with ductal cancer, a difference that was not statistically significant after adjusting for tumor size and patient age. One potential explanation for the inability of MR imaging to reduce the need for reexcision has to do with its accuracy in determining tumor size. Although multiple studies have shown that MR imaging is more accurate than mammography in determining tumor size, the degree of precision of measurement, and the ability to translate the imaging findings to the amount of tissue removed in the operating room, may be insufficient to see a reduction in margin positivity. For example, Grimsby and colleagues compared tumor size as determined by MR imaging for 190 invasive breast cancers with pathologic size measurements and found that MR imaging estimated size within 5 mm of the pathologic measurement in only about half of cases, overestimating the size in one-third of cases, and underestimating in 15%. As previously discussed, when serial subgross sectioning was used as the standard, MR imaging underestimated disease extent for 19% of invasive foci and a far higher percentage of DCIS foci. Because margin specimens are often subjected to detailed pathologic scrutiny, some of this disease will be identified as positive margins.