Fig. 9.1
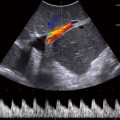
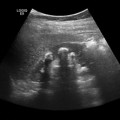
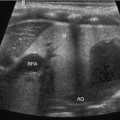
a During sonography, look for the vertebral body in the transverse plane. The aorta and inferior vena cava (IVC) will be anterior to it. The IVC is compressible. The aorta will be anechoic with a pulsating circle. In the proximal aorta, the celiac trunk can be seen with its branches (seagull sign—hepatic and splenic arteries are the wings). b Sagittal epigastric view of the aorta and its branches, celiac artery (CA), splenic artery (SA), left gastric artery (LGA), and the superior mesenteric artery (SMA). The splenic vein (SV) is visualized below the transverse cut of the pancreas (https://carmenwiki.osu.edu/display/10337/AAA)
The superior mesenteric artery (SMA) is the next major trunk off the aorta (Fig. 9.2a, b). This artery supplies the midgut with blood. The SMA first gives rise to the middle colic artery and then the right colic artery as well as the ileocolic artery. The SMA also gives rise to the several arcuate arteries that provide most of the blood to the small intestine. The middle colic artery gives rise to the marginal artery of Drummond, which supplies the transverse and the left colon.
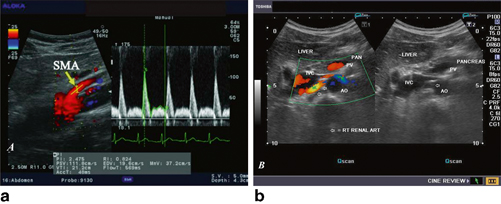

Fig. 9.2
a Color Doppler image of the aorta and the SMA. b Grayscale and color Doppler images show the origin of the normal right renal artery from the abdominal aorta (Ao), just below the origin of the superior mesenteric artery. It takes careful adjustment of the wall filter settings to remove the motion artifacts from the aortic pulsations, with appropriate pulse repetition frequency (PRF) settings to get a good color flow image of the right renal artery. The right renal artery is seen coursing behind the IVC after an antero-lateral origin from the aorta (Ao). The left renal vein (LRV) is also seen traversing anterior to the abdominal Ao and between it and the SMA. The above images are transverse sections through the epigastrium. The liver provides a good window to view these vessels (http://www.ultrasound-images.com/vascular.htm)
The left and right renal arteries also originate from the aorta, most commonly inferior to the SMA and superior to the inferior mesenteric artery (IMA), though there is wide anatomic variation here as well. Most often the renal arteries are single, but they can be double or have early and varied branching. The adrenal glands receive their blood supply from suprarenal arteries that branch directly off of the aorta as well, usually superior to the main renal arteries.
Inferior to the SMA are the gonadal arteries, which supply the testicle or ovary, and the IMA. The IMA arises proximal to the bifurcation of the aorta. The main branches of the IMA are the left colic artery, which anastomoses with the aforementioned marginal artery of Drummond, and the colosigmoid artery. Distal to the take off of the colosigmoid is the origin of the rectosigmoid artery. The superior rectal arteries are the final branches of the IMA.
The main venous drainage systems of the abdomen are the inferior vena cava (IVC) and portal vein (PV). The superior and inferior mesenteric veins drain the intestines ; the superior mesenteric vein (SMV) merges with the splenic vein to form the PV. This confluence usually occurs just dorsal to the pancreas. The gonadal veins have an asymmetric drainage, with the left gonadal vein draining into the left renal vein (LRV) and the right gonadal vein draining into the IVC directly. Similarly, the left suprarenal vein drains into the LRV, and the right suprarenal vein drains directly into the IVC above the renal veins.
Scanning Technique
For best results, exams should be done in the morning when possible. The patient should not have eaten since the previous midnight and refrain from chewing gum to reduce the amount of gaseous distention of the intestine, which can limit ultrasonography of the abdominal vessels. To reduce bowel gas further, the patient can be asked to drink some water immediately prior to the exam.
Abdominal vessels are typically imaged in several planes (Fig. 9.3). By switching the orientation of the probe, it is possible to measure the diameter of the vessels, the origin of vessels, and their angles as they take off from the abdominal aorta. Use of pulse-wave and color Doppler ultrasound allows measurement of flow in the vessels, with the measurement of velocity, resistive index, and turbuluence of flow. Color Doppler waveforms can provide information about the direction and quality of blood flow through the vessels. Waveforms can distinguish between the arterial and venous blood flow.
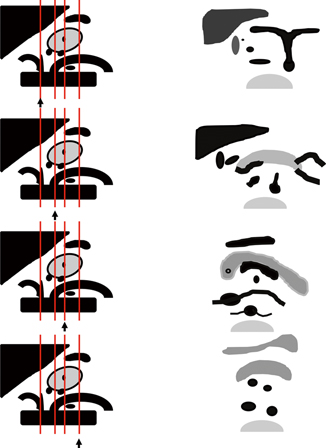

Fig. 9.3
Schematic representation of the abdominal vessels and their relationships to abdominal viscera. Left: Sagittal view of abdominal aorta, celiac, SMA, and renal artery origins and the pancreas, liver, and spleen with splenic artery and vein. Right: Each panel from top to bottom corresponds with a schematic of the ultrasound images that would be seen with the probe positioned in the line marked with the arrow in the left panel
Malrotation and Midgut Volvulus
Intestinal malrotation refers to the incomplete rotation of the small intestine in utero. It can result in midgut volvulus, or the complete torsion of the small bowel around the SMA axis, causing acute obstruction requiring prompt surgical intervention. Early recognition of symptoms—bilious emesis—and appropriate, prompt imaging can lead to earlier diagnosis and operation, improving outcomes. The anatomical repositioning of the SMA and SMV during malrotation lends itself to sonographic and especially Doppler diagnosis. Normally, the SMV lies right and ventral to the SMA; in malrotation, that positioning is switched. A large study of 337 patients demonstrated that left/right reversal had a higher sensitivity and specificity for malrotation than ventral/dorsal switching [1–6]. It is possible to have malrotation of the intestines without SMA/SMV reversal, and SMA/SMV reversal can exist without malrotation. In addition this SMA/SMV reversal can be persistent after a Ladd’s procedure [7, 8]. The best early study, a prospective comparison of US versus UGI in 427 children, found SMA/SMV reversal to have a 70 % sensitivity, 96 % specificity, 62 % positive predictive value, and 97 % negative predictive value [9].
An additional sonographic sign consistent with malrotation is the “Whirlpool sign” [10] . This finding reflects the clockwise rotation of the SMV around the SMA axis as a part of the malrotation. A subsequent study confirmed both the importance of the clockwise orientation (with counterclockwise orientation commonly reflecting nonspecific hemorrhagic enteritis) and the value of this finding [11]. Early reports documented sensitives in the 90th percentiles and specificities approaching 100 % [12, 13]. While specificities remain high, reports of whirlpool sign in patients without malrotation have also emerged [14]. Figure 9.4 contains an example illustration [15].
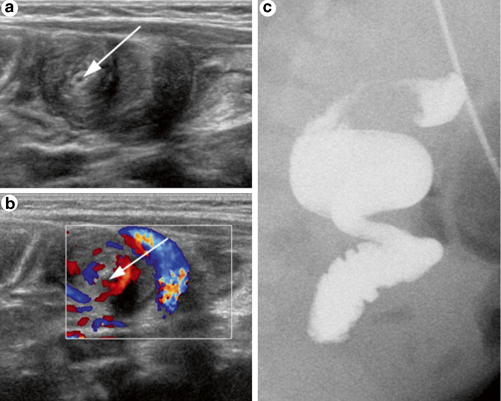

Fig. 9.4
Midgut volvulus diagnosed using ultrasound (US) in an 83-day-old boy evaluated for hypertrophic pyloric stenosis. Gray-scale (a) and Doppler US (b) demonstrate the “whirlpool sign” of the peripheral swirling superior mesenteric vein (SMV) around the SMA (arrow). UGIs (c), which followed the US, shows the corkscrew sign of midgut volvulus, which was confirmed during surgery [15]
An alternative strategy for diagnosing malrotation with ultrasound involves assessing the position of the third portion of the duodenum (D3). Normally, D3 rests in the retroperitoneum. In malrotation, D3 will be intraperitoneal and anterior to the mesenteric vessels [16]. Sonographic demonstration of a retroperitoneal D3 effectively excludes the diagnosis of malrotation and thus that of midgut volvulus [17–19] . This technique is simple and efficient for diagnosis of malroatation in newborns.
Ultrasound has some limitations. Bowel gas can be problematic. Most series report an inability to perform sonography on around 15 % of the patients due to bowel gas interference [20]. Multiple studies have demonstrated a significant false-negative rate with ultrasound, corresponding to sensitivities in the 70–80 % range [21]. Given these detractions and the serious consequences of a volvulus diagnosis, clinicians strongly suspicious of malrotation with volvulus should continue to order upper gastrointestinal radiological studies (UGIs), even after a negative ultrasound. However, the low-cost, noninvasiveness, lack of radiation, and strong specificity of several sonographic features—especially whirlpool sign —make ultrasound an excellent screening modality where positive findings may allow the surgeon to skip additional diagnostic steps and proceed directly to the operating room.
Compression Syndromes
Compression syndromes are products of anatomic variants in abdominal vasculature that result in compression of intra-abdominal organs to produce a pattern of symptoms. The difficult evaluations for these conditions have been simplified by the use of abdominal US of the vessels in question.
Median Arcuate Ligament Syndrome
Median arcuate ligament syndrome (MALS), also called the celiac artery compression syndrome and Dunbar syndrome, occurs when the median arcuate ligament compresses the celiac axis and celiac ganglion, typically producing abdominal pain. It was first identified by Harjola in 1963 [22]. The median arcuate ligament connects the right cura and the left cura of the diaphragm, forming the anterior border of the aortic hiatus.
Inexpensive, noninvasive, rapid, and without the risks of contrast and radiation, Doppler ultrasound provides an effective alternative to the angiography and CT-angiography. Two prospective trials in adults comparing the Doppler Ultrasound to the lateral abdominal aortography confirmed the accuracy of this modality in diagnosing the condition. The more recent investigation confirmed 100 % sensitivity of the Doppler ultrasound for identifying clinically significant (> 70 %) celiac artery stenosis. Specificity, positive predictive value, and negative predictive value were 87, 57, and 100 %, respectively [23, 24]. A later retrospective analysis on children and adolescents further substantiated the suitability and value of the Doppler ultrasound in diagnosing MALS.
For the examination, patients should fast for a minimum of 8 h prior to the study. Given the vague clinical presentation, the entire mesenteric system should be evaluated. Lower frequency (in the range of 3–7.5 MHz) and harmonic imaging provide clearer images. All velocity measurements should be obtained with angle correction of less than 60° [25]. Wolfman et al. have demonstrated the importance of acquiring both supine and erect Doppler images, noting a tendency of pathological values to normalize when children are positioned erect. The stage of the respiratory cycle dramatically affects the results, with much higher peak velocities observed at peak expiration than peak inspiration due to the dorsal movement of the aorta upon inhalation. One study in adults suggested that 73 % of the patients with end-expiration celiac artery stenosis observed via ultrasound demonstrated at least partial resolution of the pathology at end-inspiration [26, 27]. Consensus for the proper moment in the respiratory cycle to examine the patient has not been established, with authors alternatively opting for either the best-case or worst-case ultrasonagraphic results. Examinations require fewer than 20 min for experienced ultrasongraphers to complete.
While normal blood flow velocities for adults and neonates are available, such data are not well-established for children, and no rigorously verified criteria for defining MALS in the pediatric population exist. For adults, prospective studies have shown that peak systolic velocities greater than 200 cm/s in the celiac artery (or no flow detected) correlate with at least 70 % stenosis of the vessel, with 75 % sensitivity and 89 % specificity [25]. Most subsequent publications have adopted these standards, even for children, and have demonstrated their reliability in diagnosing the MALS [24]. The largest series of children with MALS (n = 59) entertained more liberal criteria, including greater than twofold acceleration in flow between the aorta and celiac artery, but a lack of confirmatory data prevents us from endorsing this expanded definition. Figure 9.5 illustrates the findings in MALS.
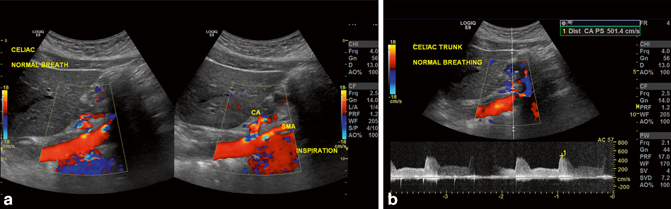

Fig. 9.5
Median arcuate ligament syndrome (MALS). a the angle between the celiac axis and the SMA is seen and measured erect and supine as well as in deep inspiration. b The velocity measured is > 200 cm/s
When appropriate, surgical intervention can proceed via either open or laparoscopic means. For either approach, the use of intraoperative ultrasound can confirm the efficacy of the repair and the resolution of the stenosis by showing decreased peak velocities in the celiac artery [28]. Thus, for screening, diagnosis, and post-treatment verification, Doppler ultrasound is a highly effective, inexpensive modality well-suited for the pediatric population.
Superior Mesenteric Artery Syndrome (SMAS)
SMAS (also referred to as Wilkie’s disease, cast syndrome, arterial mesenteric duodenal compression) occurs when the SMA and aorta entrap and compress the third portion of the duodenum. It was first noted by Rikitansky in 1842 and more completely described by Wilkie in 1927 [29].
Ultrasound has now assumed an important role in screening for the condition. A 2005 controlled prospective study in adults clearly demonstrated the ability of ultrasound to match CT scans in accurately identifying the vascular features of this syndrome [30, 31]. Exams are performed after an overnight fast and during the expiratory phase of respiration. Low MHZ probes provide the best images. There was no significant difference between conducting the exam in the supine or standing positions, although the lateral position was less sensitive.
Ultrasound can help diagnose SMAS by assessing the angle between the aorta and the SMA (Fig. 9.6a and b) as well as the distance from the aorta to the SMA at the level of the duodenum [32]. In adults, these values have been well studied, and while measurements vary, the literature generally considers a normal angle to be between 25 and 60° and a normal distance to be between 10 and 28 mm. These values in children have recently been studied in 205 consecutive pediatric abdominal CT scans in patients presenting without suspicion for SMAS. This series showed the mean angle to be 45.6 ± 19.6° [33]. The few published cases demonstrating the utility of ultrasound to diagnose SMAS in young patients with otherwise unexplainable abdominal pain have used adult measurements [34]. However, the study of 205 pediatric abdominal CT scans revealed that over 20 % of the cases demonstrated angles less than 8°—values that meet diagnostic criteria for SMAS, yet the patients denied any symptoms of the syndrome [33]. Moreover, other data show significant inter-operator variability with Doppler measurements of the SMA, challenging the reliability of test. Clearly, clinicians must have a high index of suspicion to evaluate for SMAS and use imaging to confirm clinical assessment rather than solely relying on angles and distances. At present, ultrasound serves well as a screening modality for SMAS, but CT scans ought to be obtained to confirm the diagnosis and clarify the anatomy, particularly if operative intervention is planned.
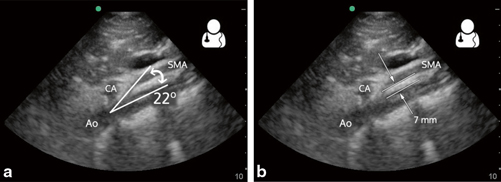

Fig. 9.6
Superior mesenteric artery syndrome (SMAS). A normal aortomesenteric angle is approximately 45°, and an aortomesenteric angle of 6–25° confirms the diagnosis. This angle can be readily measured on ultrasound. a To measure the aortomesenteric angle, first obtain a sagittal view of the aorta and delineate landmarks: Ao, CA, and SMA. Measure the SMA angle as it takes off from the aorta. b A normal aortomesenteric distance is 10–28 mm. An aortomesenteric distance < 8–10 mm suggests SMA syndrome in the appropriate clinical setting [32]
Nutcracker Syndrome
Nutcracker syndrome refers to an array of symptoms associated with the compression of the LRV. It is crucial to distinguish the syndrome from nutcracker phenomenon, which refers to anatomical and radiological evidence of such constriction without any corresponding symptoms. Anatomists have recognized compression of the LRV between the aorta and SMA since the 1930s [35, 36], but the condition of nutcracker syndrome was not elucidated until the 1970s [37]. Children suffering from the disease report flank and/or abdominal pain, gross hematuria, and varicoceles; some present with asymptomatic proteinuria or microhematuria [38]. Today, ultrasound has an important role in establishing the diagnosis.
Prior to ultrasound, renal venography with pressure gradients established the presence of the disease, with pressure differentials of greater than 3 mm Hg between the LRV and vena cava confirming the disease. This methodology remains the gold-standard, if rarely implemented. (Normal pressure differentials in children have not been established, forcing clinicians to rely on adult parameters). The invasiveness, radiation, and contrast load, not to mention cost, render this technique unappealing, especially in children. In 1986, Wolfish et al. proposed using ultrasound as an alternative [39]. Specifically, they suggested a 50 % increase in the diameter of the LRV between the renal hilum and the aorta compared to the segment between the aorta and vena cava reflected compression. The first recorded case of the nutcracker syndrome diagnosed by ultrasound occurred in 1988 in Japan using Wolfish’s criteria [40]. Further Japanese studies established clear guidelines for the diagnoses nutcracker phenomenon based on LRV dilation ratios [41, 42], but additional investigation revealed normal LRV diameter varied too much in children to rely on it for diagnostic purposes [43, 44].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree