Chapter Outline
Analysis of the Functional Aspects of Swallowing
Adaptation, Compensation, and Decompensation
Functional Aspects of Zenker’s Diverticulum
Functional Changes After Radiation Therapy
Neurologic Disease and the Pharynx
Analysis of the Functional Aspects of Swallowing
In reviewing the pharyngeal swallow, the slow motion, reverse, and stop-frame capabilities of videofluorography are essential. With these capabilities, the movement of individual structures can be analyzed, first in isolation and then in combination with other structures, including the tongue, palate, epiglottis, hyoid bone, larynx, and cricopharyngeus. Esophageal peristalsis should also be evaluated, but discussion of this subject is beyond the scope of this chapter.
Familiarity with the anatomy, radiographic anatomy, and physiology of the pharynx and related structures is a necessity for abnormalities to be appreciated. Any lack of movement or abnormalities indicating compensation or decompensation must be noted.
Two important principles must be considered when reviewing pharyngeal studies:
- 1.
Dynamic imaging is vital. Pharyngeal contraction occurs much faster than esophageal contraction (12-25 cm/s vs. 1-4 cm/s), which is why dynamic imaging is essential when examining the pharynx. In addition, an abnormality such as a web or laryngeal penetration may be visible on only one or two frames at 30 frames/s.
- 2.
The entire swallowing chain must be examined. Such extensive examination is necessary because the level of symptoms is not a reliable indicator of the site of the abnormality, several lesions could be causing dysphagia, and esophageal disease may result in pharyngeal disease.
Neurophysiologic Control of Swallowing
Swallowing involves the close cooperation of many muscles, six cranial nerves (trigeminal, facial, glossopharyngeal, vagus, spinal branch of accessory, and hypoglossal) and the first, second, and third cervical nerves (through the ansa cervicalis). Afferent sensory information is integrated in the swallowing center in the brain stem, and efferent signals originate in the motor ganglia of the cranial nerves; movements are then effected peripherally.
The vagus nerve (cranial nerve [CN] X) supplies motor efferent fibers to all the intrinsic pharyngeal muscles (constrictors, palatopharyngeus, and salpingopharyngeus), except the stylopharyngeus, which is supplied by the glossopharyngeal nerve (CN IX). The vagus nerve also supplies motor efferent fibers to all the palatal muscles, except the tensor veli palatini, which is supplied by the trigeminal nerve (CN V). The trigeminal nerve also supplies the anterior digastricus and mylohyoideus. The facial nerve (CN VII) supplies the posterior digastricus and stylohyoideus. Although the vagus nerve carries the efferent fibers that innervate the striated pharyngeal musculature, most of these fibers probably emerge from the brain stem in the bulbar part of the accessory nerve (CN XI).
Pharyngeal branches of the glossopharyngeal and vagus nerves and rami of the sympathetic trunk and superior cervical ganglion form a plexus in the connective tissue outside the constrictor muscles (the pharyngeal plexus). In this plexus, autonomic (parasympathetic and sympathetic) and afferent and efferent branchial fibers intermingle and branch into the muscles and the mucosal lining. Damage to this plexus can produce dysphagia.
Pharyngeal sensation, including sensation of the tonsil and postsulcal part of the tongue, appears to be mediated by the glossopharyngeal nerve. This nerve also supplies motor innervation to the stylopharyngeus and parasympathetic secretomotor fibers to the parotid gland.
Functional Components of Swallowing
Oropharyngeal Phase
Tongue and Palate.
Swallowing begins with the lips engulfing the bolus ( Figs. 15-1 and 15-2 ). The bolus is then manipulated by the tongue and teeth until it is judged “swallowable.” Two positions of the bolus preparatory to swallowing have been identified: the tipper type (in which the bolus is held in the midline groove of the tongue) and the dipper type (in which the bolus is held anteriorly underneath the tongue in the floor of the mouth).


The back of the tongue blade and soft palate form a seal that prevents premature leakage into the pharynx before swallowing (see Figs. 15-1A and 15-2A ). Weakness, atrophy, or resection of the tongue or soft palate can lead to aspiration before swallowing because of leakage of the bolus into the open, unprotected larynx. Viewed in the frontal position, unilateral leakage indicates decompensation on one side only.
As the bolus is propelled into the oropharynx by an upward backward movement of the tongue, the soft palate elevates to a right angle to appose the posterior pharyngeal wall, with Passavant’s cushion moving anteriorly to complete the seal of the palatopharyngeal isthmus. Passavant’s cushion results from focal contraction of the upper fibers of the superior pharyngeal constrictor muscle ( Fig. 15-3 ; see also Figs. 15-1B, 15-2B, 15-10A, 15-11A, and 15-11B ). Tongue thrust (involving first the blade and then the base of the tongue), combined with pharyngeal constriction and intrabolus pressure, result in bolus propulsion.

Pharynx.
The constrictor stripping wave can be observed at the tail of the bolus in the lateral position as a progressive forward movement of the posterior pharyngeal wall (see Figs. 15-1C to F and 15-2C to E ). In the frontal position, the wave can be appreciated as the lateral walls of the pharynx converge to the midline, completely obliterating the pharyngeal cavity behind the bolus. Unilateral weakness results in asymmetry on the frontal view, with one side moving to the midline and the other remaining still or bulging during the swallow. Sometimes, the contracting normal side can displace the bolus across to the atonic side, so the bolus passes down the paralyzed side. The contracting normal side may be misinterpreted as a mass, whereas the abnormality is actually on the noncontracting bulging side. Contraction of the constrictor muscles may be affected by intrinsic disease of the pharyngeal muscles (e.g., polymyositis), neuromuscular disorders (e.g., amyotrophic lateral sclerosis, cerebrovascular accident [CVA]), or local factors (e.g., scarring, radiation, cervical spine disease). Large osteophytes in the cervical spine may hinder or prevent epiglottic tilt. Diseases that restrict laryngeal elevation (e.g., radiation, head and neck surgery) may compound the problem.
Laryngeal Dynamics and Hyoid Elevation
Respiration is suspended during swallowing and resumes after swallowing. As the bolus enters the oropharynx, the larynx begins to elevate, the hyoid bone moves upward and forward, and the true vocal folds, false vocal folds, and laryngeal vestibule close inferiorly to superiorly, with the vestibule closing last. Laryngeal elevation begins simultaneously with elevation of the hyoid bone but continues for a short time after the hyoid bone has reached its peak elevation. Laryngeal movement can be appreciated by observing the hyoid bone rising to appose the angle of the mandible. There is an excellent review of laryngeal dynamics by Curtis. A direct correlation exists between hyoid bone excursion and bolus volume, with larger volumes producing more elevation. Hyoid bone elevation may occur in one step (20%) or in two steps (80%), whereas descent occurs in one step, simultaneously with return of the epiglottis to the upright position.
A recent review of the literature of hyoid and laryngeal kinematics has revealed a large variability in the values reported for superior hyoid displacement, varying from 5.8 to 25.0 mm. Anterior displacement similarly ranged from 7.6 to 18 mm. Another recent study has correlated hyoid displacement with cervical spine height (measuring from the anteroinferior border of C4 to the anteroinferior border of C2) and compared findings in men and women. When adjusted for height, the upward movements of the hyoid and larynx were similar in magnitude in men and women.
Epiglottic Tilt
Epiglottic tilt deflects food and liquid into the lateral food channels away from the larynx; when completely inverted, the epiglottis covers the laryngeal aperture. In many people, this movement occurs in two steps; the first movement (to a horizontal position) is probably a passive one caused by elevation of the hyoid bone and the second movement (to complete inversion) is probably caused by contraction of the thyroepiglotticus. In a minority of people, the epiglottis fails to invert, tilting only to the horizontal or oblique position. In the frontal view, the completely inverted epiglottis produces a seagull-shaped filling defect. Flow of the bolus into the lateral food channels may also produce a flow defect, or pseudomass.
Cricopharyngeal Opening
Pharyngeal constriction must be coordinated with cricopharyngeal relaxation and opening. The cricopharyngeus must relax and open completely to allow unimpeded bolus passage. Opening of the lumen at the pharyngoesophageal junction results from several actions: (1) cricopharyngeal relaxation; (2) superior and anterior movement of the larynx, resulting in traction on the anterior wall of the lumen; (3) pharyngeal constriction, producing thrust; and (4) intrabolus pressure, producing thrust. Thus, cricopharyngeal prominence is often observed in patients with pharyngeal paresis or a frozen larynx. In such cases, the pharyngeal milieu, rather than the muscle itself, is abnormal.
Radiographically, one normally observes luminal opening, not cricopharyngeal relaxation; a radiographic report therefore should not comment on cricopharyngeal relaxation but only on cricopharyngeal opening. Cricopharyngeal achalasia is a manometric term referring to complete failure of cricopharyngeal relaxation, an unusual entity. Other common conditions associated with cricopharyngeal prominence include Zenker’s diverticulum, advanced age, gastroesophageal reflux, and other esophageal motility disorders (e.g., diffuse esophageal spasm, achalasia; Fig. 15-4 ). The resulting luminal compromise may be minimal or marked. In severe cases, there may be a horizontal bar. The luminal narrowing can be treated by endoscopic bougienage.

Pharyngeal Residue
The normal swallow should clear most of the bolus from the mouth and pharynx. (An exception is high-density barium designed to coat the gastrointestinal [GI] tract). A study by Dejaeger and colleagues in 1997 studied mechanisms involved in postdeglutition residue in older adults and found that retention in the valleculae and piriform sinuses was related to markedly reduced pharyngeal shortening, a low tongue-driving force, and diminished amplitude of pharyngeal contraction. Retention in the valleculae only is related to a low tongue-driving force and in the piriform sinuses only is related to reduced pharyngeal shortening.
Prevention of Aspiration
Many mechanisms protect the larynx from aspiration (see Figs. 15-3 and 15-11 ). Laryngeal elevation and closure of the vocal folds, arytenoid cartilages, and laryngeal vestibule all contribute, as does epiglottic tilt. Thus, a frozen larynx can result in aspiration.
If aspiration is observed, all factors that prevent aspiration should be analyzed and the following questions asked:
- •
Does the epiglottis tilt, and does the larynx (hyoid bone) elevate adequately?
- •
Do the vocal cords and laryngeal vestibule close?
It should also be noted when the aspiration occurs in relation to swallowing. Laryngeal penetration or aspiration through the vocal folds may occur during swallowing, before swallowing (premature leakage from the mouth), or after swallowing (overflow aspiration of a retained bolus in the pharynx, or regurgitated of refluxed material). The timing and causes of aspiration have potential therapeutic implications.
Another important observation is whether the aspirated material precipitates a cough; this needs to be conveyed to the referring physician in the radiologic report. Some patients with chronic aspiration are silent aspirators, presumably because of loss of laryngeal sensation. Bedside evaluation of these patients for aspiration is notoriously unreliable. In fact, the so-called bedside evaluation underestimates the possibility of aspiration in many patients.
The important role that the mouth can play in aspiration was underestimated until two important studies highlighted the role of oral decompensation alone in aspiration. Feinberg and Ekberg studied a group of 50 patients with known aspiration; they found that aspiration was the result of oral decompensation alone in 23 patients, a combination of oral and pharyngeal decompensation in 17 patients, and pharyngeal decompensation alone in 10 patients. The same authors studied oral decompensation in 75 patients who survived a near-fatal choking episode. Abnormalities were found on videography in 58 of these 75 patients. Oral stage dysfunction was the predominant finding in 32 patients, with pharyngeal abnormalities in 19 and cricopharyngeal abnormalities in 28.
Reduced vertical excursion of the hyoid or larynx may contribute to incomplete airway closure and the risk of aspiration. Reduced anterior displacement may result in reduced upper esophageal sphincter (UES) and cricopharyngeal (CP) opening, contributing to piriform sinus residue.
Adaptation, Compensation, and Decompensation
The pharynx is an extremely flexible organ that must adapt to its various functions, including respiration, speech, and swallowing. In addition, the pharynx adapts to different stimuli, such as the size and consistency of the bolus and temperature of ingested liquids and food; the pharynx compensates when one of its parts is defective. The process of adjustment of normal swallowing to different stimuli is called adaptation . The pharynx must adjust to the bolus, which may vary in volume, temperature, consistency, viscosity, and elasticity. The effect of different boluses can be seen with videofluoroscopy by watching the difference between a swallow of thin liquid barium and a swallow of the same volume of barium paste.
The effect of a carbonated bolus on the physiology of the swallow has been investigated. Carbonated liquids reduced penetration, aspiration, and pharyngeal retention, and pharyngeal transit time was shorter.
When signs of compensation are visible on dynamic studies, swallowing is already impaired. Some types of compensation for impaired swallowing may be conscious and voluntary. For example, a patient may change the types of foods eaten, perhaps omitting solid food and substituting puréed food, or may even restrict the diet to liquids only. Certain postures such as flexing the neck (chin tuck) or turning the head may help a patient swallow more effectively, reducing laryngeal penetration or aspiration and clearing any retained bolus.
Swallowing can be considered to have five distinct stages, each of which has a characteristic pattern of compensation and decompensation. The five stages include the following:
- 1.
Control of the junction of the mouth and pharynx (palatoglossal seal; Fig. 15-5 )
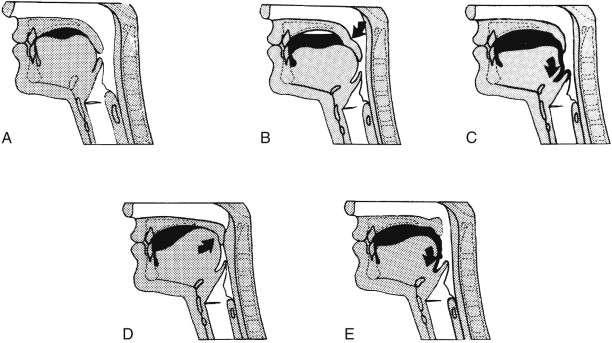
Figure 15-5
Control of the junction of the mouth and pharynx.
A. Leakage from the back of the mouth is prevented in the normal patient when the soft palate abuts the posterior portion of the tongue. B. Deficiency of the tongue caused by atrophy, weakness, incoordination, or postsurgical defect may be compensated by downward displacement of the palate ( arrow ), with the palate “kinking” to appose the tongue. D. Conversely, palatal deficiency is compensated by upward displacement of the tongue ( arrow ). Note that the bolus is held further forward in the mouth under these circumstances. C, E. Decompensation, in which there is premature leakage of oral contents into the pharynx ( arrow ), with the potential for overflow aspiration.
(From Buchholz DW, Bosma JF, Donner MW: Adaptation, compensation, and decompensation of the pharyngeal swallow. Gastrointest Radiol 10:235–240, 1985.)
- 2.
Closure of the palatopharyngeal isthmus ( Fig. 15-6 )

Figure 15-6
Closure of palatopharyngeal isthmus during swallowing.
A. Normal closure, in which the soft palate has elevated to a right angle to abut Passavant’s cushion. B. Deficiency of the pharyngeal palate may be compensated by increasing convergence of the pharyngeal constrictor muscles ( arrow ), resulting in a very prominent Passavant’s cushion. C. Decompensation results in nasopharyngeal regurgitation through the palatopharyngeal isthmus ( arrow ).
(From Buchholz DW, Bosma JF, Donner MW: Adaptation, compensation, and decompensation of the pharyngeal swallow. Gastrointest Radiol 10:235–240, 1985.)
- 3.
Compression of the bolus ( Fig. 15-7 )

Figure 15-7
Compression of the bolus.
A. Normal compression. B. Deficiency of the constrictor muscles may be compensated by increased upward and posterior displacement of the tongue and larynx ( arrow ). D. Deficiency of the tongue in bolus compression may be followed by more anterior displacement of the constrictor wall ( arrow ), resulting in a very prominent pharyngeal stripping wave. C, E. Decompensation caused by inadequate bolus compression results in bolus retention in the valleculae and piriform sinuses after swallowing, with the potential for overflow aspiration.
(From Buchholz DW, Bosma JF, Donner MW: Adaptation, compensation, and decompensation of the pharyngeal swallow. Gastrointest Radiol 10:235–240, 1985.)
- 4.
Closure of the larynx ( Fig. 15-8 )

Figure 15-8
Closure of the larynx.
A. The normal condition, in which the larynx is elevated and closed, and the epiglottis ( arrow ) is completely tilted down to cover the entrance to the laryngeal aditus. B. With deficiency of epiglottic tilting or glottic closure, there may be increased upward and anterior displacement of the larynx ( arrow ). Under these circumstances, the arytenoid masses may be enlarged as a sign of compensation (not illustrated). C. Failure of compensation results in penetration of bolus ( arrow ) into the laryngeal vestibule, and even through the incompletely closed cords, with aspiration.
(From Buchholz DW, Bosma JF, Donner MW: Adaptation, compensation, and decompensation of the pharyngeal swallow. Gastrointest Radiol 10:235–240, 1985.)
- 5.
Opening of the pharyngoesophageal segment ( Fig. 15-9 )

Figure 15-9
Opening of the pharyngoesophageal segment.
A. Normal opening of pharyngoesophageal segment. B. Deficiency of upward laryngeal displacement, which contributes to opening of the pharyngoesophageal segment, results in flexion of the neck and/or forward thrusting of the jaw during swallowing. C. Failure of compensation results in poor pharyngoesophageal segment opening, with retention of the bolus in the piriform sinuses and the risk of overflow aspiration.
(From Buchholz DW, Bosma JF, Donner MW: Adaptation, compensation, and decompensation of the pharyngeal swallow. Gastrointest Radiol 10:235–240, 1985.)
During the first stage, for example, downward placement of the soft palate may compensate for deficiencies of the tongue (resulting from atrophy, weakness, or surgical resection). Conversely, upward displacement of the tongue may compensate for weakness of the soft palate (see Figs. 15-5 to 15-9 for compensation occurring during other stages of swallowing). Another compensatory phenomenon is the development of a deeper pharyngeal stripping wave, which may be observed in the presence of a partially obstructing lesion in the cervical esophagus, such as a web or cricopharyngeal bar.
Any radiographic findings of compensation must be communicated to the referring physician so the patient can be informed that swallowing is impaired The patient can then be instructed to take additional care when eating or drinking quickly—for example, in a restaurant or in other social situations.
Functional Aspects of Zenker’s Diverticulum
Zenker’s diverticulum is a pulsion diverticulum located at the level of the pharyngoesophageal junction; the most common site is between the oblique and horizontal fibers of the cricopharyngeal muscle through a triangular area known as Killian’s dehiscence ( Fig. 15-10 ). It is a posterior diverticulum that may fill during or after swallowing and, if large, may flop to one side or the other. Once swallowing is completed, the diverticulum may empty back into the pharynx, filling the piriform sinuses. This often precipitates a second swallow but also places the patient at risk for overflow aspiration.

The pathogenesis of Zenker’s diverticulum remains unclear, but incoordination between pharyngeal contraction and cricopharyngeal opening may be a contributing factor. Manometric and cineradiographic studies have demonstrated premature sphincter closure in some patients with Zenker’s diverticulum. Other studies performed in patients with fully developed diverticula, however, have shown that the timing of pharyngeal contraction and cricopharyngeal relaxation may be normal.
Cook and associates demonstrated decreased compliance of the UES in a group of patients with Zenker’s diverticulum, in whom inadequate sphincter opening resulted in increased intrabolus pressure. Shaw and co-workers subsequently demonstrated that with cricopharyngeal myotomy and pouch ablation, the intrabolus pressure fell and full UES opening was restored. They also observed structural abnormalities in muscle strips of the cricopharyngeus in 14 patients with Zenker’s diverticulum. They found type 1 fiber predominance and greater fiber size variability. The muscles also demonstrated fibroadipose tissue replacement and fiber degeneration. These studies suggest that decreased UES compliance may have a major role in the development of Zenker’s diverticulum.
Gastroesophageal reflux may also contribute to the development of Zenker’s diverticulum. In an analysis of 67 patients with Zenker’s diverticulum, 63 were found to have esophageal pathology such as gastroesophageal reflux, spasm, or hiatal hernia (T. Karaho, unpublished data, 1998).
Radiologically, Zenker’s diverticulum is usually associated with prominence of the cricopharyngeus or a cricopharyngeal bar. Esophageal disease may produce prominence of the cricopharyngeus, perhaps with decreased compliance, which in turn may raise intrapharyngeal and intrabolus pressure, necessitating more forceful contraction, with eventual bulging through a congenitally weak area. Further studies are necessary (including those addressing the early developmental stages of Zenker’s diverticulum) before the pathophysiology can be fully understood.
Interrelationships
Esophageal Distention and the Cricopharyngeus
Intraluminal foreign bodies lodged in the hypopharynx or upper esophagus may produce cricopharyngeal spasm. In cats, it has been shown that stimulation of afferent receptors in the hypopharynx or upper cervical esophagus evokes reflex contraction and spasm in the cricopharyngeal muscle, with an increase in intraluminal pressure in the sphincter segment. Similarly, esophageal distention by liquids or an intraluminal balloon in humans results in elevated pressure in the sphincter segment.
Gastroesophageal Reflux and the Cricopharyngeus
There are variable reports in the manometric literature as to whether acid bathing the esophagus produces a rise in pressure in the UES. In the 1970s, studies in human volunteers using perfused catheters reported elevated pressures in the sphincter segment after infusion of the esophagus with saline or acid. The closer the infusion was to the UES, the greater the increase in pressure. In the late 1980s, a study using a modified sleeve sensor compared UES pressures in normal persons and patients with esophagitis ; this study reported no UES response to acid reflux or acid perfusion. Another manometric study using solid-state catheters has shown many interactions among the lower esophageal sphincter, gastroesophageal reflux, intraesophageal pressure changes, and changes in pressure in the UES.
Several reflexes have been described, primarily by Shaker and co-workers :
- 1.
UES contractile response to gastroesophageal reflux. Torrico and colleagues compared UES pressure changes in normal controls and patients with reflux esophagitis ; most of the controls and patients with reflux esophagitis had a significant increase in UES pressures in response to reflux episodes.
- 2.
UES contractile response. Mechanical stimulation of the pharynx in cats and water stimulation in humans results in an increase in UES resting tone. As little as 0.1 mL of water produced this reflex.
- 3.
Esophagoglottal closure reflex. Esophageal distention during gastroesophageal reflux could overwhelm the UES, with the potential for overflow aspiration. A reflex has been described in humans and cats in which esophageal distension resulted in reflex closure of the glottis.
- 4.
Pharyngoglottal adduction reflex. An injection of minute amounts of water into the pharynx resulted in brief closure of the vocal folds. This was thought to be a protective reflex to prevent aspiration.
Swallowing-Related Reflexes
Many cardiorespiratory reflexes are related to swallowing, resulting from laryngeal, pharyngeal, or esophageal stimulation. Examples include syncope, change in heart rate, apnea, and bronchoconstriction leading to asthma. A review of swallowing-related reflexes is referenced for further reading.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree