Name:
_______________________________________________
City:
_______________________________________________
Region:
________________________________________________
Country:
________________________________________________
Hospital, University or Private:
________________________________________________
1. How many patients with newly diagnosed localised PCa do you see at your institution per year?
__________________________________________________________________
2. Which treatment strategies do you offer to patients with localised PCa at your institution ?
Surgery :
__________%
EBRT:
__________%
Brachytherapy:
__________%
HIFU:
__________%
Cryotherapy:
__________%
Active surveillance
__________%
Other modalities (please specify):.
__________%
(__________)
3. Do you treat patients with focal therapy for localised PCa in your centre?
Y
N
(if NO, proceed to Question 4)
Focal Photodynamic therapy:
__________%
Focal Brachytherapy:
__________%
Focal Laser-induced interstitial thermotherapy:
__________%
Focal HIFU:
__________%
Focal cryotherapy:
__________%
Other focal treatment modalities (please specify):
__________%
(_______)
4. For how many patients on AS at your institution would you estimate focal therapy to be an option?
__________%
5. If there are no patients on AS at your institution,
(a) Would you be open to the idea?
Y
N
(b) Would patients accept AS in your centre?
Y
N
6. If there are no patients on focal therapy at your institution,
(a) Would you be open to the idea?
Y
N
(b) Would patients accept focal therapy at your centre?
Y
N
(c) Would patients accept focal therapy better than AS?
Y
N
7. Are you aware of sources (websites, references, etc.) for information on focal therapy for localised PCa in your country?
Y
N
If yes, please specify:
________________________________________________________________________
Thanks for your cooperation,
Prof. Van Poppel and Dr. Joniau
Table 3.2
Number of key opinion leaders per country to whom a questionnaire was sent
Country | Number of key opinion leaders to whom a questionnaire was sent |
AT | 4 |
BE | 4 |
CH | 5 |
DE | 15 |
EL | 3 |
ES | 6 |
FR | 12 |
IT | 16 |
LT | 1 |
NL | 7 |
PT | 2 |
RO | 1 |
RU | 2 |
SE | 4 |
SI | 1 |
TR | 2 |
UK | 10 |
PSA Screening
Widespread use of PSA screening has increased the incidence of PCa and led to both over-diagnosis and over-treatment of PCa. Regional differences have been observed in PCa incidence and mortality rates,1 but a relationship between a trend towards lower mortality for PCa and intensity of screening is not clear. The extent of benefit and harm with PSA screening is under continuous debate. A benefit is that early detection by screening allows men to choose for more management options and may provide peace of mind to men who want to do all they can to prevent or treat the situation if cancer is discovered. A disadvantage is that current treatments including RP and RT result in considerable side effects in many men such as erectile dysfunction, incontinence and bowel dysfunction 7.
Two studies assessed the effect of PCa screening on mortality. A significant 20% relative reduction in PCa mortality due to PSA screening was recently shown in the large European Randomised Study for Screening of Prostate Cancer (ERSPC) study within 9 years of follow-up (risk ratio for death from PCa = 0.80, 95% CI = 0.65–0.98; adjusted P = 0.04) 8. No such effect was observed in the smaller US National Cancer Institute-sponsored Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial within 7–10 years of follow-up (risk ratio = 1.13, 95% CI = 0.75–1.70) 9. The Göteborg randomised population-based PCa screening trial with a median follow-up of 14 years showed a mortality reduction of 44%. For strictly screened patients, PCa mortality was even reduced by 56% 10. To resolve the PCa screening debate, further analyses will be needed from the ERSPC and PLCO studies, as well as from others, such as the Prostate Cancer Intervention Versus Observation Trial (PIVOT) in the USA (NCT00007644), 11 and the Prostate Testing for Cancer and Treatment (PROTECT) trial (ISRCTN20141217) 12 and Comparison Arm for Protect (CAP) cluster randomised controlled trial (ISRCTN92187251) 13 in the United Kingdom (UK).
In Europe, PCa testing is less common than in the USA where guidelines for PSA screening exist. In the USA, PSA use between 2004 and 2010 declined slightly by 3.0 percentage points and 2.7 percentage points among men aged 40–54 and 55–74 years, respectively 14. PSA testing among men aged >75 years initially declined slightly following the recommendations by the US Preventive Services Task Force in 2008 and continued to decline after the publication of the 2 PCa screening studies in 2009 8,9 examining the effect of screening on PCa mortality 14.
A recent publication reports PCa mortality in the USA and UK in 1975–2004. It shows a fourfold greater decline in PCa mortality in the USA, compared with the UK since 1992. This period corresponded with a much higher uptake of PSA screening in the USA. In 2001, 57% of men in USA aged ≥ 50 years reported having a PSA test within the previous year, 15 while in the UK, only 6% of men aged 45–84 years were tested 16,17. Localised disease is treated more aggressively in the USA than in the UK. Whether PCa screening or other factors (such as improved treatment) should be accounted for the declining mortality rate beyond 2000 is still a subject of much debate 15. In France we have observed a notable increase in PCa screening. In 2005, 58% of general practitioners (GPs) recommended PCa screening for their male patients aged 50–74 years; in 2008, this figure was 65% (OR = 1.32, 95% CI = 1.04–1.66) 18. This trend was observed before the release of the results of the 2 PCa screening studies 8,9.
Aggressive PCa screening is the norm uniformly throughout the USA, while in Europe PCa screening approaches differ amongst countries.
Stage and Grade Migration
A study compared the stage migration patterns between European (n = 5,739) and American (n = 5,611) patients treated with RP from 1988 to 2005. The proportion of American patients with organ-confined disease after RP increased to approximately 80% in 2001, followed by a subsequent decrease to 60% in 2005. Moreover, this trend was accompanied by an increase in patients with high-grade PCa (Gleason score ≥ 7) at clinical and surgical pathology. Conversely, the stage migration pattern in Europe has been reported to follow a sustained increase, resulting in 75% of patients having organ-confined disease after RP in 2005. This trend was accompanied by a decrease in patients with high-grade PCa (Gleason score ≥ 7) and an increase of the rate of Gleason sums of 6 at the expense of all other Gleason sums 19,20. A recent study showed an inverse stage migration trend in 8,916 European men with PCa who were treated with RP between 2000 and 2009. The proportion of low-risk patients dropped from 66% in 2004 to 35% (P = 0.016) in 2009. A decrease of favourable disease (organ confinement and Gleason 3+3 grade) from 53 to 17% (P = 0.008) and an increase in the number of patients with non-organ-confined PCa from 19% in 2003 to 33% in 2009 (P = 0.008) were observed. The restriction of the analyses in the present study to a single tertiary-care centre could limit the generalizability of the results. The increase of non-organ-confined disease after RP in European patients is a trend also seen in the USA. It could be related to changes in patient selection, the growing acceptance of multimodality treatment for locally advanced tumours and the broader use of alternative treatment regimens to RP, such as brachytherapy, focal therapy and AS protocols in low-risk patients with PCa 20.
Active Surveillance
Active surveillance (AS) represents an active decision not to treat the patient immediately for PCa but to follow him closely using serum PSA levels and repeat prostate biopsies. The purpose is to switch to radical treatment when there is evidence of disease progression, determined by PSA doubling time and deteriorating histopathological factors on repeat biopsy 21.
Rationale for Active Surveillance
Analysis of data from the Cancer of the Prostate Strategic Urologic Research Endeavour (CAPSURE) database showed a significant increase in the percentage of patients with low-risk tumour characteristics from 27.5% in 1990–1994 to 45.8% in 2004–2006 22. About 50% of men with PSA screen-detected PCa are “over-diagnosed”, that is, their cancer will never cause symptoms during their lifetime, even if left untreated 23-25. The rationale of AS is to reduce over-treatment in patients with clinically confined low-risk PCa, without giving up treatment with curative intent. This is in contrast to the watchful waiting (WW) strategy where curative treatment is avoided because of advanced age or co-morbidity of the patient and which is described for decades as an observation strategy with the use of palliative treatment for symptomatic progression. AS also aims to overcome the radical treatment-related morbidity and the impact on a man’s lifestyle. Other studies that support the rationale of AS are those published by Albertsen 26-28. Men with low-grade PCa have a minimal risk of dying from PCa during 20 years of management by observation or androgen withdrawal therapy alone (Gleason score of 2–4, six deaths per 1,000 person-years, 95% CI, 2–11) 26. Results following conservative management of clinically localised PCa diagnosed in the PSA era are better than outcomes in studies from earlier eras. Considering favourable 10-year outcomes, patients with a life expectancy of less than 10 years may wish to consider an AS or WW protocol 27. A recent study reported that most men with localised PCa and older than 65 years with either no or one co-morbidity will survive at least 10 years, whereas men with two or more co-morbidities have a substantial risk of dying as a result of a competing medical hazard within this time frame. Patients and clinicians should consider using co-morbidity-specific data to understand the risks posed by localised PCa and by competing medical hazards 28.
Outcomes
According to the guidelines of National Institute for Health and Clinical Excellence (NICE) (2008) in the UK, AS is an option for men with low- or intermediate-risk localised PCa that involves close monitoring to target curative treatment to those who would benefit most 29. The guidelines of NICE (2008) on AS for PCa are based on 5 studies that are limited to localised PCa (stage T1-T2) with PSA <15 ng/mL suitable for curative treatment and which provide the best evidence of the importance of AS 30-35. After a mean follow-up of 40 months, 8–33% of patients experienced progression 30. The National Comprehensive Cancer Network (NCCN) guidelines suggest AS for men with very low risk PCa when life expectancy <20 years or men with low risk PCa when life expectancy <10 years. The patient’s PCa risk profile, age, and health should be taken in consideration. The NCCN panel believes that there is an urgent need for further clinical research regarding the criteria for recommending AS 36. The EAU guidelines (2011) on AS for PCa are based on seven studies 5,37-42 which all confirm that in carefully selected patients with low-risk PCa, there is a very low rate of progression and cancer-specific death and only a small number of patients need delayed radical treatment. In these studies, rate of progression ranged from 9 to 36% and rate of CSS ranged from 97.2% to 100%. However, longer follow-up (another 5–7 years) will be required in order to obtain definitive results 43. A recent AS study in the USA including 230 patients with mean age of 63.4 years showed that if criteria for AS are strictly defined to include only patients with Gleason score 6, tumour volume ≤ 20% in one or two biopsy cores and PSA levels ≤ 10, few patients are likely to require treatment. In total, 86% remained on AS for a mean follow-up of 44 months and 14% were treated for a mean follow-up of 33 months 44. The encouraging results of AS studies led to phase III studies comparing AS with radical treatment: PRIAS, PROTECT, START. These studies will provide additional insight into the outcome of AS and the required follow-up strategies.
The Prostate cancer Research International Active Surveillance (PRIAS) study is an international prospective observational study initiated by the Rotterdam section of the ERSPC 8 and the Department of Urology of the Erasmus Medical Center in Rotterdam (Dutch Trial Register NTR1718, http://www.prias-project.org) to validate the management of PCa with AS 45. Candidates for this protocol-based AS programme are men fit for curative therapy with asymptomatic T1c/T2 PCa, with a PSA level of ≤10 ng/mL at diagnosis, PSA density (PSA/prostatic volume) <0.20 ng/mL/mL, Gleason score of ≤3 + 3 = 6 and one or two positive biopsy cores (http://www.prias-project.org). The PRIAS study is entirely web based and presents the largest prospectively analysed cohort of patients in an AS programme, and has good protocol compliance. Participating centres are located in the Netherlands, Finland, Italy, France, Belgium, Austria, Spain, Germany and Canada. Short-term results of the first 500 (>950) participants, when applying the strict PRIAS inclusion and follow-up protocol, show 25% of men stopping AS and switching to active treatment within 2 years after diagnosis. Switching to active treatment is mainly because 20% of standard repeat biopsies at 1 year after diagnosis show adverse characteristics, which is independent of the PSA doubling time. The median (25–75th percentile) follow-up after diagnosis was 1.02 years (0.6–1.5 years). The 2-year survival rate free from active therapy was 73%. In total, 82 men changed to active therapy during follow-up, 24 of whom had RP. After RP, 4 of 24 men had T3 disease and 12 men had Gleason score >6. A longer follow-up is warranted to evaluate mortality outcomes 45,46. Another trial to evaluate the efficacy of AS and radical treatment (RP or radiation) is the PROTECT trial in the UK with a follow-up of 10–15 years. Patients were recruited until 2008. Major outcomes will be reported around 2016. The National Cancer Institute has launched the Standard Treatment Against Restricted Treatment (START) trial, a randomised controlled trial which aims to study differences between AS and radical treatment (RP, external radiation or brachytherapy) in men with early PCa. It will enrol 2,130 men with low-risk localised PCa in Canada, the USA and the UK. PIVOT is a multi-centre randomised controlled trial in the USA that compares RP to WW in men with clinically localised PCa. The Scandinavian Cancer Group Study Number 4 (SPCG-4) is a randomised controlled study that compared the long-term outcomes of RP with WW. Recently, Bill-Axelson et al. reported in an update of this trial, a cumulative incidence of PCa death at 15 years of 14.6% for the men assigned to RP and 20.7% for those assigned to WW. The survival benefit with RP was similar before and after 9 years of follow-up, was observed also among men with low-risk PCa and was confined to men aged <65 years. The fact that active treatment has better results than WW in men aged <65 years can be seen as a warning for blind introduction of AS strategies in clinical practice. The finding that the effect of RP is modified by age has not been confirmed in other studies of RP or EBRT 47.
Patient Selection for AS for PCa
Results of the ongoing AS studies are eagerly awaited. In the meantime, earlier study results suggest that AS is a therapeutic option for PCa tumours with low-risk of progression. According to the EAU guidelines, 43 low-risk PCa for which AS is primarily recommended is defined as follows: PSA ≤10 ng/mL, biopsy Gleason score ≤ 6, ≤ 2 positive biopsies, ≤ 50% cancer per biopsy, cT1c-2a.
Improvement of AS Procedures
Appropriate AS requires validated markers to distinguish patients with indolent tumours and those with aggressive potential suitable for radical treatment. Several questions remain unanswered. Should inclusion in AS studies be based on nomogram use? Should inclusion and/or follow-up be based on new biomarkers? Should we use individual AS protocols depending on co-morbidity and age? What is the quality of life on the long term? Is delay in radical treatment dangerous? What are the costs? PRIAS will be an important source of information with respect to application and safety of AS. With limited patient numbers available for analysis, no significant differences were found in adverse intermediate outcomes between immediate RP and delayed RP in the Swedish section of the ESRPC. The delayed RP group may be subject to a selection bias. Prospective evaluation of AS protocols is necessary. 48 A recent cost analysis using the SPCG-4 study revealed that during a median follow-up of 12 years, RP was associated with a 34% (P < 0.01) higher cost than WW 49.
Low Acceptance
The NCCN guidelines recommend AS for men with very low-risk PCa when life expectancy <20 years or men with low-risk PCa when life expectancy <10 years 36. An analysis of the CAPSURE database between 1999 and 2004 showed that 16.4% of men presenting with localised PCa met the criteria for very low-risk disease (Epstein surveillance criteria; PSA <10 ng/mL, cT1 or T2a, PSA density <0.15, fewer than 1 or 3 biopsy cores positive and absence of Gleason pattern 4 and 5 on biopsy). Only a small subset (9.0%) of patients eligible for AS actually chose this form of treatment, suggesting that AS may be underused in the management of very low-risk PCa 50. Approximately a third of patients on AS require treatment 5,51,52. Improved patient counselling and education are urgently needed. A study on contemporary men with low-risk PCa managed at a large US academic institution revealed an acceptance of AS in 57% of the low-risk patients 53.
For patients who have difficulty to accept AS and want to do something, rather than just wait, there may be the use of a 5-alpha reductase inhibitor. It seems that 5-alpha reductase inhibitors may enhance AS in PCa. A Canadian single-institution retrospective cohort study compared nearly 300 patients taking a 5-alpha reductase inhibitor versus no 5-alpha reductase inhibitor while on AS for PCa. Patients were half as likely to show pathologic progression if they were taking a 5-alpha reductase inhibitor 54. However, more investigation is needed. Some physicians have negative attitudes towards AS. This may be due to the concern about the inaccuracies of clinical staging and grading and the lack of randomised controlled studies evaluating the AS strategy. Other reasons for poor acceptance of AS are the upgrading and upstaging of patients who underwent RP, but who would theoretically have been candidates for AS. Jeldres et al. performed a European validation of the Epstein criteria for insignificant PCa in a cohort of 366 patients. Unfavourable final histopathology was found at RP, which consisted of either pathologic Gleason 7 disease (n = 88, 24%), or non-organ-confined pathologic stage (n = 30, 8.3%). Caution is advised when treatment decisions are based only on Epstein criteria for insignificant PCa as these criteria my underestimate the true nature of PCa in as many as 24% of European patients 55.
Patient’s Decision to Participate in AS for PCa and Psychologic Impact
The use of a decision aid for patients with localised PCa in clinical practice has a positive impact on the consultation and the treatment decision-making process 56. A questionnaire of the University of Miami to assess factors that contributed to the patient’s decision to participate in AS for PCa revealed that physician’s influence was the greatest contributor (73%). Potential side effects of incontinence (48%) and erectile dysfunction (44%) associated with radical therapy were other reasons for choosing AS. The questionnaire response was 57% (105/185) 57. Similarly, the use of a questionnaire in 150 Dutch men participating in the PRIAS study showed that 62% of men choosing AS favoured this strategy because “quality of life and lifestyle” are not altered. The most frequently reported advantage of AS was the delay of side effects. The most frequently reported disadvantage of AS was the risk of disease progression. The questionnaire was returned by 86% (129/150) of patients. Van den Bergh and colleagues found that patients with early PCa enrolled in their AS protocol had adequate knowledge of PCa and realistic expectations about AS 58.
Controversy exists on the psychological burden that might be associated with AS. Analysis of data from CAPSURE showed that anxiety can be an independent factor that drives patients on AS towards active treatment 59. A cross-sectional study in the UK assessed the prevalence of anxiety and depression in patients with localised PCa managed by AS compared with those receiving immediate radical treatment. AS was not associated with greater psychological burden than more immediate treatment for PCa 60. A Canadian publication suggests the development of strategies to alleviate anxiety and uncertainty if AS is to become more widely adopted. Additional information and psychological support resources are required for men on AS 61. In the Scandinavian study SPCG-4, anxiety and depression were more common in the WW group than in the RP group 62. Recently, only 10% of the Dutch men surveyed and participating in the PRIAS study experienced psychological morbidity associated with having an “untreated tumour” 58. More recently, the same authors reported that Dutch men participating in the PRIAS study showed significant but clinically irrelevant decreases in the mean scores of the State Trait Anxiety Inventory (P = 0.016) and the Memorial Anxiety Scale for Prostate Cancer fear of progression subscale (P = 0.005). They concluded that anxiety and distress remain favourably low during the first nine months of AS 63.
Status of Active Surveillance in Europe
The questionnaire reveals that surgery remains the most commonly used radical treatment option in Europe for localised PCa. The literature shows that in the USA, relatively few physicians recommend and few patients accept AS 64,65.
The percentage of patients who were offered AS in the ten European countries is presented in Fig. 3.1. The country with the highest percentage of AS for localised PCa is Switzerland (39%). It should be noted that institutional differences within a country may vary widely according to the key opinion leaders’ interest in the treatment modality with percentages of patients on AS ranging from 0 to 75%. Figure 3.2 gives an overview of the percentage of patients on AS per European key opinion leader. We also examined the respondents’ attitude about AS. As all key opinion leaders offered AS, although some in a very small percentage of patients, the questions “if there are no patients on AS at your institution would you be open to the idea and would in that case patients accept AS in your centre” remained unanswered.
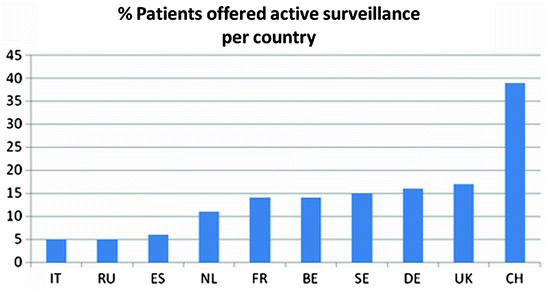
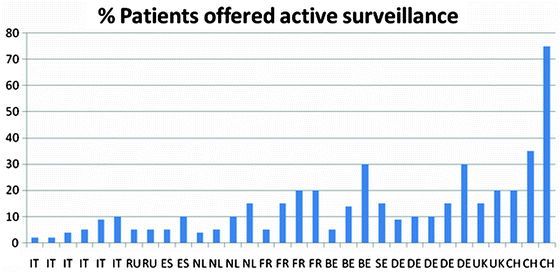

Fig. 3.1
Percentage of patients offered active surveillance per European country

Fig. 3.2
Percentage of patients offered active surveillance by European key opinion leader
Perhaps in the next years, AS will become “much more accepted” and men will be more comfortable with the idea of postponing treatment.
Focal Therapy
Focal therapy for PCa is currently under development as a therapeutic strategy for patients with low-volume, localised PCa. It aims to offer a “middle ground” between AS and radical therapies by achieving long-term cancer control while only eradicating the malignant areas and preserving a maximum of healthy prostate tissue. Minimising damage to surrounding structures may reduce side effects associated with radical therapies such as impotence, incontinence and rectal toxicity.
Rationale for Focal Therapy of Localised PCa
The concept of organ-sparing treatment has proven its efficacy for selected tumours of the breast, skin, bladder and kidney. The concept of focal therapy for PCa has been promoted by the same arguments as for AS, such as recent stage migration towards low-risk PCa, potential overtreatment of clinically insignificant tumours with radical therapy and associated side effects, but also by under-treatment of patients improperly selected for AS, the relatively low acceptance of AS because of the anxiety associated with delayed treatment of cancer and the now available technological advances needed for local target ablation of the prostate.
Several modalities have been proposed for PCa focal therapy, including high-intensity focused ultrasound (HIFU), cryotherapy, photodynamic therapy (PDT), laser-induced interstitial photothermal therapy and brachytherapy. All these treatment modalities are able to cause localised focal necrosis within the prostate of a predetermined size in a relatively controlled way. Currently, there are two transrectal devices that can treat the prostate with HIFU: the Ablatherm® device (Edap-Technomed, Lyon, France) and the Sonablate® 500 (Focus Surgery, Indianapolis, IN, USA). At present, focal therapy using cryosurgery has the most experience. Further testing this technique in randomised, multicenter studies is required. Another approach is irreversible electroporation using the NanoKnife® (AngioDynamics, Queensbury, NY, USA).
Patient Selection for Focal Therapy of Localised PCa
Candidate selection remains the major challenge of implementing focal therapy in clinical practice. The Consensus Panel including key opinion leaders from Europe and North America at the end of “the 2nd International Workshop on Focal Therapy and Imaging in Prostate and Kidney Cancer” (Amsterdam, the Netherlands, June 10–13, 2009, http://www.focaltherapy.org) achieved agreement on the patient selection following a transperineal template prostate mapping biopsy, inclusion of patients with low-risk, small-volume PCa (e.g. Gleason 6, clinical stage ≤ T2a), estimated life expectancy of 10 or more years, the fact that imaging is probably going to play a significant role in the coming years and the unknown long-term effects on urinary and potency/erectile functions 66.
Identifying Patients Who May Be Candidates for Focal Therapy
Ideal candidates for focal therapy seem to be men with unifocal lesions, unilateral lesions or index lesions (biologically most aggressive lesions) accompanied by non-index lesions with very low-risk features. Up to a third of the patients with localised PCa have unilateral disease that may be suitable for hemi-ablation of one lobe 67-69. At present, we do not have a definition of clinically significant disease. The key determinants are volume and grade, though both are closely related. Today, we are unable to predict clinically significant unifocal or unilateral PCa with sufficient accuracy. However, using aggressive biopsy schemes, possibly in combination with magnetic resonance imaging (MRI), the probability of missing a large tumour can be reduced 70. It has been demonstrated that significant volume tumours larger than 0.5 cm3 (diameter <9–10 mm) gives rise to disease progression 71. Eighty percent of secondary non-index lesions are <0.5 cm3 72




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree