Multimodal MR imaging provides valuable information in the management of patients with acute ischemic stroke (AIS), with diagnostic, therapeutic, and prognostic implications. MR imaging plays a critical role in treatment decision making for (1) thrombolytic treatment of AIS patients with unknown symptom-onset and (2) endovascular treatment of patients with large vessel occlusion presenting beyond 6 hours from the symptom onset. MR imaging provides the most accurate information for detection of ischemic brain and is invaluable for differentiating AIS from stroke mimics.
Key points
- •
Multimodal MR imaging provides valuable information in the management of patients with acute ischemic stroke (AIS), with diagnostic, therapeutic, and prognostic implications.
- •
MR imaging plays a critical role in treatment decision making for (1) thrombolytic treatment of AIS patients with unknown symptom-onset and (2) endovascular treatment of patients with large vessel occlusion presenting beyond 6 hours from the symptom onset.
- •
MR imaging provides the most accurate information for detection of ischemic brain and is invaluable for differentiating AIS from stroke mimics.
Introduction
Until 2015, intravenous (IV) thrombolysis was the only approved treatment of patients with acute ischemic stroke (AIS) presenting within 4.5 hours from symptom onset. Over the past 5 years, AIS treatment has been revolutionized with addition of endovascular treatment (EVT) to the approved treatment options and by extending the treatment window out to 24 hours.
Neuroimaging played a significant role in this rather rapid evolution and in adopting the concept of a tissue clock over time for treatment decision making. The main objectives of neuroimaging in patients with AIS are to determine the extent of initial ischemic core and identify the location and extent of intravascular clot as well as the presence and extent of penumbra (hypoperfused tissue at risk for infarction). ,
Although computed tomography (CT) is the most commonly used imaging modality for patients with AIS, due mostly to its wide availability and faster acquisition time, MR imaging is an appealing alternative due to its high diagnostic accuracy (sensitivity and specificity) for acute ischemia, which can be used for swift treatment decision making and to stratify stroke mimics as a major source of confusion in emergency departments. Gradual decrease in the prevalence of MR imaging contraindications due to new MR imaging–safe pacemaker and stent devices and improved accessibility, which may be afforded by introduction of new small-footprint MRI, potentially could cast a new horizon for broadened use of MR imaging in AIS patients in the future.
This article reviews the role of MR imaging for the evaluation of patients presenting with AIS. How a multiparametric MR imaging stroke protocol is performed and can be used in management of AIS is reviewed. Some of the clinical applications, including MR imaging–based treatment decision making according to the updated 2018 American Heart Association (AHA)/American Stroke Association (ASA) guidelines, are reviewed. Finally, how MR imaging stroke protocol can be used in differentiating stroke mimics and work-up of patients with cryptogenic strokes is reviewed.
MRI stroke protocol
Image Acquisition
At the authors’ institution, in absence of contraindication, MR imaging is the default imaging modality for patients presenting with suspicion of AIS. A comprehensive MRI stroke protocol consists of the following: (1) diffusion-weighted imaging (DWI); (2) fluid-attenuated inversion recovery (FLAIR); (3) gradient-echo (GRE); (4) MR angiography (MRA); and (5) perfusion imaging.
Improvements in MR imaging technology, including fast sequence design, such as echo planar imaging (EPI), and fast imaging tools, such as parallel acquisition algorithm, have resulted in significant improvements in efficiency of MR imaging for acute stroke imaging. A comprehensive MR stroke protocol now can be obtained in approximately 6 minutes, rivaling those of CT algorithm. By addressing the long acquisition time, the major drawback of MR imaging for acute stroke imaging is related to a more complex workflow in comparison to CT. Improvement in workflow can be achieved by having a multidisciplinary stroke team arrange an organized approach from hospital arrival to angio suite, minimizing the delays between steps.
Some of the measures that can accelerate the MR imaging workflow include (1) activation of stroke code with notification sent to MRI to prepare the MRI suite ahead of arrival, (2) assigning a stroke nurse and emergency department technologist with defined and specific roles, (3) simplifying the MRI clearance form, and (4) use of MRI–compatible devices in the emergency department, including MRI-compatible electrocardiographic leads and table for transport before imaging. Using some of these measures, Screening with MRI for Accurate and Rapid Stroke Treatment (SMART) investigators were able to reduce the door-to-needle time by approximately 40% for thrombolytic treatment of patients with AIS. Adopting similar strategies, another group of investigators showed that MRI can be used effectively for patient selection in EVT, with the admission–to–groin puncture time of 68 minutes (median) comparable to what was achieved in clinical trials, such as in the Solitaire with the Intention for Thrombectomy as Primary Endovascular Treatment for Advanced Ischemic Stroke (SWIFT PRIME) study.
Image interpretation
Diffusion-weighted imaging
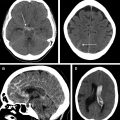
The major advantage of using MRI for acute stroke imaging is having a DWI sequence as the most sensitive imaging modality for early ischemia ( Fig. 1 ). DWI has level I evidence for assessment of ischemic core , and is now the recommended sequence in patient selection for late window thrombectomy.

The advent of DWI has redefined stroke syndromes, such as acute ischemic infarction and transient ischemic attack (TIA), from an all-or-none process to a dynamic and evolving process. Approximately 40% of patients with a clinically defined TIA can have restricted diffusion indicative of ischemic infarction, a finding that has led to an ASA-endorsed proposal to revise the definition of TIA from time-based to tissue-based criteria. Another useful point of information obtained from DWI is that the pattern of the DWI abnormalities provides insight into the underlying etiology and stroke subtypes, such as lacunar or embolic etiologies.
Fluid-attenuated inversion recovery
The major applications of FLAIR in acute stroke imaging are (1) a complementary role to GRE for detection of intracranial hemorrhage ( Fig. 2 ) and, in particular, for identifying subtle subarachnoid hemorrhage, (2) FLAIR hyperintense vessel sign as a marker of slow flow and collaterals; in the setting of proximal arterial occlusion, FLAIR high signal intensities in the blood vessels within the subarachnoid spaces have been attributed to hemodynamic impairment, representing slow retrograde flow in leptomeningeal collateral ; and (3) determining the age of the infarction, which probably is the most important application. As a rule, the prevalence of lesion visibility on FLAIR increases as time passes from the stroke onset and up to 93% of acute stroke lesions could have positive FLAIR findings at greater than 6 hours. Following successful completion of the WAKE-UP (Efficacy and Safety of MRI-based Thrombolysis in Wake-up Stroke) trial FLAIR, in addition to DWI (DWI-FLAIR mismatch), now is recommended to identify acute so-called call wake-up stroke (discussed later). A faster variety of FLAIR imaging (EPI-FLAIR) can be obtained in one-third the acquisition time of conventional FLAIR but with similar diagnostic performance and has been used successfully for patient treatment decision making.

Gradient-echo
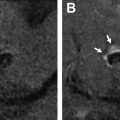
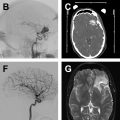
The common applications of GRE are (1) detection of acute intracranial hemorrhage in pretreatment screening (see Fig. 2 ), where GRE alone or in combination with FLAIR has excellent diagnostic accuracy in detection of acute intracranial hemorrhage comparable to CT , ; (2) detection of intraarterial clot seen as increased local susceptibility signal, known as susceptibility vessel sign , —this is defined as a dark blooming artifact visible on T2∗-weighted GRE images because of the magnetic susceptibility effect of deoxygenated hemoglobin in the red blood cells trapped in an occluded artery ( Fig. 3 ); and (3) detection of hemorrhagic transformation in AIS patients treated with thrombolytic or mechanical revascularization therapies ( Fig. 4 ). Although CT is equally sensitive for detection of parenchymal hematomas, petechial hemorrhages are detected by GRE with significantly higher sensitivity. The clinical importance of these microbleeds when chronic is unknown and current AHA/ASA guidelines do not recommend MR imaging–GRE screening to withhold thrombolytic treatment. Faster variety of GRE imaging, such as EPI-GRE, provides similar diagnostic performance to conventional GRE but only a fraction of the acquisition time.


MR angiography
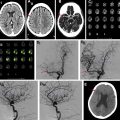
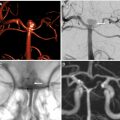
Vascular imaging is an essential component of any stroke imaging protocol. In potential candidates for EVT, a noninvasive vascular study of intracranial circulation (level Ia) and extracranial arteries (level IIa) is recommended during the initial imaging evaluation. Inclusion of neck and extracranial vascular tree provides useful additional information about the aortic arch anatomy, tortuosity of the supra-aortic arteries, and possibility of tandem proximal stenosis, to facilitate treatment planning and achieving safer and faster reperfusion in patients with acute stroke (see Fig. 3 , Fig. 5 ). In AIS patients with suspicious of arterial dissection, fat-saturated T1-weighted images can be obtained to look for intramural hematoma (see Fig. 5 ).

The commonly used MRA techniques in stroke imaging include noncontrast time-of-flight (TOF) technique and CE-MRA. The sensitivity of CT angiography (CTA) and MRA in comparison to the gold standard of digital subtraction angiography ranges from 87% to 100%, with CTA having a slight edge over MRA. , In the setting of acute stroke imaging, CE-MRA is advantageous over TOF-MRA because it provides fast and efficient delineation of intracranial arteries and collateral circulation beyond the point of occlusion afforded by T1 shortening effect of gadolinium injection and with similar performance to CTA. TOF-MRA can be limited by longer acquisition times and inability to assess distal arterial stenosis due to spin saturation and phase dispersion secondary to slow, in-plane, or turbulent flow. , Recent studies have shown higher diagnostic accuracy for CE-MRA than TOF-MRA in detection of arterial stenosis and occlusion in patents with AIS. , With the advent of fast imaging tools, CE-MRA of the entire supraoptic arteries now can be obtained during a 20-second acquisition time. Combining CE-MRA and dynamic susceptibility contrast (DSC) perfusion has been shown clinically feasible, , to provide results similar to those accomplished by CTA and CT perfusion (CTP).
MR perfusion
Due to fast acquisition time and relative ease of postprocessing, bolus DSC has been used as a robust and widely accepted technique to measure cerebral perfusion in patients with acute stroke. The major applications are as follows:
- 1.
Defining ischemic penumbra: in patients with AIS and following a cerebral arterial occlusion, there is a developing ischemic core and surrounding hypoperfused brain tissue that potentially is at risk of infarction in the absence of reperfusion. This potentially salvageable tissue is referred to as ischemic penumbra. Timely revascularization, on the other hand, can salvage the ischemic penumbra from infarction. Multiple parametric maps can be obtained from DSC perfusion to assess hemodynamic compromise, including mean-transit time, time-to-maximum (Tmax), cerebral blood flow, and cerebral blood volume (CBV). Among these, Tmax has been used consistently for assessment of ischemic penumbra and volume of Tmax greater than 6 seconds is now considered a measure of tissue-at-risk and a target for thrombectomy therapies (see Fig. 4 ).
- 2.
Identifying DWI-negative TIA patients: DWI-negative patients, who account for greater than 50% of all TIA patients, remain a source of uncertainty and great clinical challenge. Many of these patients may have transient cerebral ischemia, where the degree of hemodynamic compromise did not reach the severity required to cause restricted diffusion. Perfusion imaging can be used to detect regions of hypoperfusion or postischemic hyperperfusion in approximately 25% to 30% of these patients, providing additional evidence to confirm a footprint of ischemia that otherwise would go undetected.
Clinical applications
Treatment Decision
Thrombolytic treatment
For AIS patients presenting less than 4.5 hours from onset, in absence of contraindication, thrombolytic treatment can be administered if there is (1) no intracranial hemorrhage and (2) no large ischemic core. The latter is defined as lack of clearly hypoattenuating region on initial CT rather than subtle hypodensities on noncontrast CT that can contribute toward a lower ASPECTS (Alberta Stroke Program Early CT Score) in the most updated AHA/ASA guidelines. There is no APECTS cutoff for exclusion of patients from receiving thrombolytic treatment. If using MR imaging for initial assessment of AIS patients who present less than 4.5 hours from the onset, IV tissue plasminogen activator can be administered safely in the absence of large infarction core on diffusion MR imaging and intracranial hemorrhage.
For patients presenting with unknown onset or wake-up strokes, there likely is a potential role for MR imaging for thrombolytic treatment selection. Unwitnessed AIS is relatively common, accounting for approximately 15%–25% of all AIS patients, who before 2019 would not have received reperfusion therapies. Many of these patients, however, presumed to be after 4.5 hours from onset, still have salvageable brain tissue that is maintained by collateral circulation. With the recent positive WAKE UP trial, which used DWI-FLAIR mismatch to select patients for extending the time window for IV thrombolysis, use of MR imaging (FLAIR-DWI mismatch) now is recommended (level IIa) to identify unwitnessed AIS patients who may benefit from thrombolytic treatment ( Fig. 6 ). Also, perfusion imaging has been used for extending the thrombolytic window up to 9 hours according to recently published results of Extending the Time for Thrombolysis in Emergency Neurologic Deficits trail.