In the past two decades, minimally invasive neurointerventional therapies have become the standard of care for a variety of neurologic diseases. This article reviews some of the principal areas in which these techniques can be applied successfully in acute emergencies of the brain, head, and neck.
Cerebral ischemic disease
Acute Ischemic Stroke
As mentioned in Imaging of Acute Ischemic Stroke by Carlos Leiva-Salinas and Max Wintermark, acute ischemic stroke (AIS) affects 780,000 people each year in the United States, is the third leading cause of death, and is the leading cause of long-term disability. Costs to the US health care system are in excess of $56 billion annually. Although few therapies for AIS existed before the 1990s, the past 2 decades have seen a proliferation in both noninvasive and invasive image-guided treatments for ischemic stroke. Thrombolytic therapies for AIS trace their roots to the 1980s, when the widespread availability of computed tomography (CT) scanning first permitted rapid differentiation between hemorrhagic and ischemic strokes. Intravenous tissue plasminogen activator (IV tPA) (Genentech, South San Francisco, CA, USA) was approved in 1996 for use in patients during the first 3 hours of AIS. The time window for IV tPA use was recently expanded to 4.5 hours for many AIS patients. Both small-vessel and large-vessel ischemic strokes can be treated successfully with IV tPA.
Endovascular therapies for AIS began in the 1980s with off-label use of urokinase, streptokinase, and tPA all delivered intraarterially via a microcatheter into thromboemboli occluding the large (>1 mm luminal diameter) arteries at the base of the brain. Streptokinase research was later abandoned, and intraarterial (IA) tPA became the principal endovascular AIS treatment of patients who either could not receive IV tPA ( Box 1 IV tPA exclusion criteria ), arrived too late in the time course of AIS to receive IV tPA, or whose symptoms failed to improve sufficiently following IV tPA administration. The PROACT-I (Prolyse in Acute Cerebral Thromboembolism), PROACT-II, and IMS (Interventional Management of Stroke) trials of the late 1990s and early 2000s confirmed the usefulness of IA lytic therapies in AIS up to at least 6 hours following symptom onset.
ICH or SAH
History of ICH, brain aneurysm, brain vascular malformation, or brain tumor
Recent large-vessel infarction (>one-third MCA territory loss of gray-white differentiation on NECT)
Uncontrollable hypertension (systolic blood pressure >185 mm Hg, diastolic blood pressure >110 mm Hg)
Major surgery or trauma within 3 months
Active internal bleeding within 3 weeks
Coagulopathy (platelets <100,000; partial thromboplastin time >40 seconds; international normalized ratio >1.7)
Despite the success of IA tPA in improving AIS outcome for many patients with small-vessel (lacunar) or large-vessel occlusion (LVO) strokes, many patients with LVO show persistent occlusion following microcatheter-directed thrombolysis. This finding served as the impetus for developing mechanical thrombectomy devices in the 2000s, a process that continues today. Initial experience using balloon angioplasty of intracranial large-vessel thrombosis was of mixed success, with some centers reporting favorable outcomes (vessel recanalization and improved clinical status) and others reporting a high rate of vascular perforation. In 2004, a wire-based thrombectomy device (Merci X retriever, Concentric Medical, Mountain View, CA, USA) was approved by the US Food and Drug Administration (FDA) for use in AIS. Further iterations of this device (L series and V series) added suture material to the corkscrew-shaped wire device, with improved results at intravascular clot extraction and vessel recanalization.
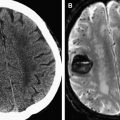
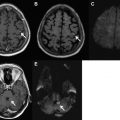
In 2008, the Penumbra device (Penumbra, Alameda, CA, USA) was approved by the FDA for treatment of LVO AIS. The Penumbra is a mechanical thrombectomy device consisting of an aspiration catheter attached to a suction pump coupled with a bulbous tipped separator wire. It is designed to macerate clot within the catheter and just beyond the catheter tip. Initial results of the Penumbra device were similar to those of the Merci device with regards to recanalization of the internal carotid artery (ICA), middle cerebral artery (MCA), and basilar artery, as well as with regards to clinical outcomes. Two case examples of mechanical thrombectomy in AIS are illustrated in Fig. 1 .
In addition to FDA-approved wire- and aspiration-based thrombectomy devices, several devices based on intracranial stent technology are currently under investigation. The fundamental observation that deployment of a stent can sometimes open a completely occluded artery has led to off-label use of intracranial stents in patients in whom IA lytics and/or approved mechanical thrombectomy devices have not achieved vessel recanalization. Balloon-expandible and open-cell self-expanding stents can be deployed permanently and closed-cell self-expanding stents can be deployed either permanently or temporarily. Transient deployment of a stent to open a vessel during stroke treatment with recapture and removal of the stent at the end of the procedure has an inherent advantage over permanent stent placement: if no stent is left in situ, then the patient does not have to be placed on long-term dual antiplatelet therapy (typically aspirin and clopidogrel). This finding is of particular importance in patients with atrial fibrillation, as the addition of multiple antiplatelet agents to a warfarin regimen can lead to an increased rate of delayed intracranial hemorrhage (ICH).
Although there is great enthusiasm for mechanical thrombectomy devices, such devices have limitations with regards to stiffness and size that prevent their safe use in vessels beyond the base of the brain. In addition, a comparison of data from the Multi-MERCI (Mechanical Embolus Removal in Cerebral Ischemia) trial and PROACT-II trial suggests that, once baseline patient enrollment characteristics are factored out, mechanical thrombectomy and microcatheter-directed IA thrombolysis seem to result in similar rates of good clinical outcome in LVO AIS.
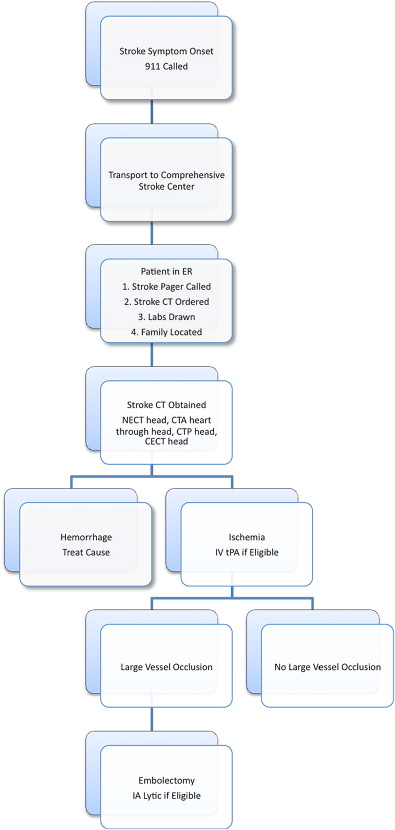
The stroke treatment algorithm and therapeutic windows currently in place at the authors’ institution are illustrated in Fig. 2 and Table 1 , respectively. Despite the time windows for AIS therapy listed in Table 1 , exceptions to these guidelines exist. Some patients have poor outcomes despite prompt institution of AIS therapy, and others have good clinical outcomes even when treated beyond 8 hours after onset of stroke symptoms. The latter is particularly important when patients awaken with stroke symptoms. In this setting, the clinical standard is to assign the time the patient was last seen well (ie, when the patient went to sleep), even if the true onset of ischemia might have been moments before the patient awoke. These wake-up strokes, as well as the more routine presentation of patients to the emergency room beyond 3 to 8 hours, has led to an intense interest in the use of magnetic resonance (MR) and CT-based physiologic imaging to better assess which patients would benefit from aggressive stroke treatment and which would not. Techniques such as MR diffusion-weighted imaging and CT perfusion imaging hold great promise in improving patient selection for AIS therapy, leading some to declare that ischemic stroke treatment is moving from a clinical paradigm of time is brain to an image-guided paradigm of physiology is brain.
| Time | Treatment Options |
|---|---|
| 0–3 h | IV tPA, IA tPA, thrombectomy |
| 0–4.5 h | IV tPA, IA tPA, thrombectomy |
| 0–6 h | IA tPA, thrombectomy |
| 0–8 h | Thrombectomy (posterior circulation or dominant hemisphere) |
| Unknown symptom onset or beyond 8 h | CT perfusion data may be helpful in clinical decision making MR perfusion and diffusion data may be helpful in clinical decision making Thrombectomy at discretion of interventionalist |
Intracranial Atherosclerotic Disease
Although LVO AIS is most commonly caused by a carotid or cardiac embolus lodging at an intracranial arterial bifurcation or at a preexisting vascular stenosis, LVO AIS can also be caused by atherosclerotic plaque rupture and in situ thrombosis. High-grade but nonocclusive intracranial stenoses can also lead to blood pressure-dependent acute, subacute, and chronic ischemic symptoms. Treatment of intracranial atherosclerotic disease (ICAD) is controversial. The WASID (Warfarin-Aspirin Symptomatic Intracranial Disease) trial recently compared aspirin with warfarin for the secondary prevention of stroke in patients with a previous brain infarct or transient ischemic attack (TIA). WASID confirmed that ICAD is a bad actor: the rate of a second stroke was similar between the aspirin and warfarin groups (approximately 20% in each at 1 and 2 years after enrollment). In addition, there was an increased risk of ICH associated with warfarin therapy. WASID has led not only to conversion of ICAD patients from warfarin to aspirin therapy but also has increased interest in endovascular treatments for ICAD. WASID also suggested that the highest risk for a second ischemic stroke is within the first 30 days after the initial ischemic stroke. Consideration of urgent revascularization of ICAD lesions following stroke or TIA thus seems to be appropriate for at least some patients.
The SAMMPRIS (Stenting vs Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis) trial is currently under way, comparing intracranial stenting (using the Wingspan stent and Gateway angioplasty balloon system [Boston Scientific Neurovascular, Fremont, CA, USA]) with medical therapy for secondary stroke prevention. This work builds on earlier uncontrolled case series and device registries describing the use of intracranial stents and coronary stents in the treatment of ICAD. Data from the Wingspan registry suggest that, whereas the 6-month restenosis rate for posterior circulation (basilar and intracranial vertebral artery [VA]) stents is approximately 20%, the restenosis rate for anterior circulation stents (supraclinoid ICA, M1 MCA) is approximately 50%. Patients receiving supraclinoid ICA stents had a 66% 6-month restenosis rate; subgroup analysis revealed an 89% 6-month restenosis rate in patients younger than 55 years. It is not certain why the supraclinoid ICA has such a high restenosis rate, although turbulence resulting from the tortuosity of the carotid siphon has been hypothesized as one potential explanation.
Cerebral Vasospasm
Cerebral vasospasm (CV) is a special form of subacute ischemia in patients with subarachnoid hemorrhage (SAH). CV is the leading cause of mortality and morbidity for patients surviving aneurysmal SAH. Approximately 15% to 20% of patients with aneurysmal SAH develop an ischemic stroke or die because of CV. Typical CV usually starts about 5 days after aneurysm rupture, peaks between 5 and 7 days after aneurysm rupture, and resolves by 10 days to 2 weeks after ictus. However, in some patients vasospasm can be severe and long lasting ( Table 2 ). Rebleeding resets the CV clock, and thus patients with multiple episodes of SAH can have prolonged courses of vasospasm. After the ruptured aneurysm is secured with surgical clip ligation or endovascular embolization, intensive care unit (ICU) management is switched from relative hypotension (to reduce risk of aneurysm rerupture) to deliberate hypertension (to overcome the ischemic effect of reduced arterial caliber caused by CV). Patients with SAH at our hospital receive HHH therapy (hypertension, hemodilution, hypervolemia) in addition to oral nimodipine to optimize cerebral blood flow and thus counteract CV. Efforts to secure the ruptured aneurysm are made as soon as possible within the first 5 days so that management can be redirected at mitigation of CV.
| Risk Factor | Effect on Risk of Vasospasm |
|---|---|
| Amount of SAH on nonenhanced CT (Fisher grade) | More blood, higher risk |
| Clinical symptom severity (Hunt and Hess grade) | Worse grade, higher risk |
| Age | Younger age, higher risk |
| Gender | Female gender, higher risk |
| Sympathomimetic drug abuse | Drug abuse, higher risk |
In patients in whom standard ICU measures to prevent vasospasm fail to prevent new or worsening focal neurologic deficits or increasing obtundation (and other causes of declining clinical status such as hydrocephalus, rehemorrhage, salt wasting, or swelling of completed infarction have been ruled out), endovascular techniques can be used successfully to treat CV. Intraarterial vasodilators such as the calcium channel blocker verapamil and the phosphodiesterase inhibitor milrinone cause preferential dilatation of cerebral arteries (vs systemic arteries) when administered by transcatheter infusion into the cervical ICAs, cervical vertebral arteries, or cerebral arteries themselves. The durability of pharmaceutical vasodilator treatments is limited. Angioplasty of the large intracranial arteries, including the supraclinoid ICA, M1 MCA, and basilar artery, is a safe and effective means for increasing cerebral blood flow. Angioplasty of other arterial segments, such as the intracranial vertebral arteries, A1 anterior carotoid artery (ACA), or P1 posterior carotid artery (PCA), is possible when these specific segments are not hypoplastic, as judged by comparison with an angiogram performed before the onset of vasospasm. At our institution, we use angioplasty for the treatment of moderate and severe vasospasm involving the large arteries at the base of the brain and intraarterial verapamil infusion for treating vasospasm involving smaller arteries or mild vasospasm of the large arteries.
Cerebral hemorrhagic disease
Aneurysmal SAH
Aneurysmal SAH afflicts about 30,000 people annually in the United States, thus constituting about 5% of all strokes and about 30% of all hemorrhagic strokes. Aneurysmal SAH constitutes 80% of cases of nontraumatic SAH, with the remaining 20% divided between perimesencephalic hemorrhage, arteriovenous fistulas (dural and pial), arteriovenous malformations (AVMs), and cavernous malformations that come to the surface of the brain. Perimesencephalic SAH is believed to be caused by a ruptured vein, and thus angiographically occult, and should therefore be a diagnosis of exclusion. Approximately half of patients with aneurysmal SAH die because of the rupture, with most of these deaths occurring within the first 2 weeks after initial hemorrhage. Mortality is front-loaded: 10% die before arrival at the hospital and 25% die within the first 24 hours after aneurysm rupture.
Ruptured brain aneurysms are highly unstable and prone to rerupture. There is at least a 4% rerupture rate within the first 24 hours, and a 30% rerupture rate within the first month. Dissecting aneurysms of the intracranial VA are even more friable and prone to rerupture than other intracranial aneurysms, with rebleeding rates within the first 24 hours exceeding 40% in some case series. Rebleeding of a brain aneurysm carries up to 70% risk of death. In ruptured aneurysms left untreated for long periods of time, risk of rerupture has been reported to decline to about 3% per year, although such natural history studies are few in an era of safe and effective surgical and endovascular aneurysm therapy.
Beyond being life-threatening, aneurysmal SAH also causes long-term disability in many survivors. Between one-third and one-half of SAH survivors have chronic cognitive deficits and concomitantly reduced quality of life. Focal neurologic deficits are less common, as is chronic hydrocephalus requiring ventriculoperitoneal shunting.
The International Subarachnoid Aneurysm Trial (ISAT) of 2143 patients with aneurysmal SAH reported that endovascular coiling of ruptured brain aneurysms geometrically amenable to either coiling or surgical clipping resulted in a lower rate of death or dependency at 1 year after treatment (23.7% for coiling and 30.4% for clipping) such that the trial was halted early because of the superiority of coiling over clipping. More recent long-term follow-up of patients from ISAT (6–14 years, mean 9 years follow-up) confirmed that 5-year survival was superior for patients receiving endovascular coiling versus surgical clipping (14% vs 11%, respectively), but that dependency rates were similar (83% independent vs 82% independent, respectively). There was an overall low (but higher) rate of recurrent SAH after 1 year for the treated aneurysm in coiled patients as opposed to clipped patients (10 vs 3, respectively). 40% of new SAH (11 of 24) were from previously untreated second aneurysms seen at the time of the initial (index) aneurysm treatment, or from entirely new aneurysms not previously detected. Based on ISAT and other data, endovascular therapy has become the treatment of choice for an increasing number of brain aneurysms in the past decade. A recent study of US hospitalizations for treatment of ruptured and unruptured intracranial aneurysms has also concluded that clipping, compared with coiling, was associated with significantly longer lengths of hospitalization and significantly higher total hospital charges for patients with both ruptured and unruptured aneurysm.
Several factors are ideally taken into account in the selection of endovascular versus surgical treatment of any given ruptured aneurysm:
- 1.
Location of the aneurysm
- 2.
Geometry of the aneurysm
- 3.
Age of the patient
- 4.
SAH clinical grade
- 5.
Patient comorbidities
- 6.
Experience of the interventionalist and surgeon
- 7.
Preferences of the patient or patient’s family.
In general, the deeper the location of the aneurysm and more brain retraction that is required to reach it surgically, the more likely that endovascular therapy offers treatment with lower risk of side effects.
Aneurysm geometry is generally defined as narrow-necked saccular, broad-necked saccular, or fusiform. Aneurysms with narrow necks that tend to self-retain coils within the aneurysm sac often are also favorable for straightforward surgical clipping. An additional aspect of aneurysm geometry that should be taken into account is overall size of the aneurysm. Aneurysms less than 2 mm in diameter can be challenging to coil because of their small size, given that the smallest detachable aneurysm coils are 1.5 mm to 2 mm in diameter. However, in a large series of aneurysms 3 mm or less in diameter receiving endovascular coiling, procedural success and complication rates were similar to coiling of larger aneurysms, with a slightly higher procedural rupture rate (mostly asymptomatic) and lower rate (5%) of need for subsequent retreatment. Giant aneurysms (25 mm or greater in diameter), which can be difficult to treat surgically because of their large size, can also be challenging to treat with primary coiling, as they often require many coils to achieve adequate aneurysm occlusion. Endovascular therapy with stents, flow diverters, or liquid embolics (see later discussion) may offer advantages over coiling alone, including a potential reduction in the overall cost of therapy.
Saccular aneurysms with broad necks often require adjuncts for successful endovascular therapy. Balloon-assisted coiling involves temporary inflation of a balloon in the parent artery to encourage coils to assume a self-sustainable stable configuration within the aneurysm. Patients with broad-necked coiled aneurysms are often placed on aspirin following the procedure to reduce parent artery embolic phenomena derived from clots forming on the large coil mass facing the parent artery. Stent-supported coiling consists of positioning a stent in the parent artery across the aneurysm neck to hold coils separately placed in the aneurysm (either through the stent or around the stent). The disadvantage of stent placement for treatment of acutely ruptured aneurysms is the requirement for dual antiplatelet therapy, including both aspirin and clopidogrel, to reduce the risk of stent thrombosis and distal thromboembolization. Although aspirin is generally well tolerated, clopidogrel may increase the risk for secondary hemorrhage during external ventricular drain (EVD) placement. However, at least one center has reported a large series of ruptured aneurysms treated with primary stent coiling without noting any increased frequency of secondary hemorrhage. Early EVD placement to relieve hydrocephalus before surgical or endovascular aneurysm occlusion is prudent for the relief of increased intracranial pressure (ICP) and has a secondary benefit of allowing potentially safer use of clopidogrel or gpIIb/IIIa inhibitors (such as abciximab or eptafibitide) in patients in whom a stent is necessary to achieve stable coil position.
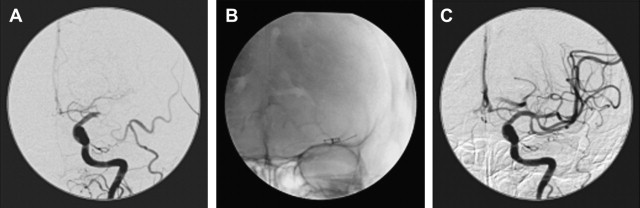
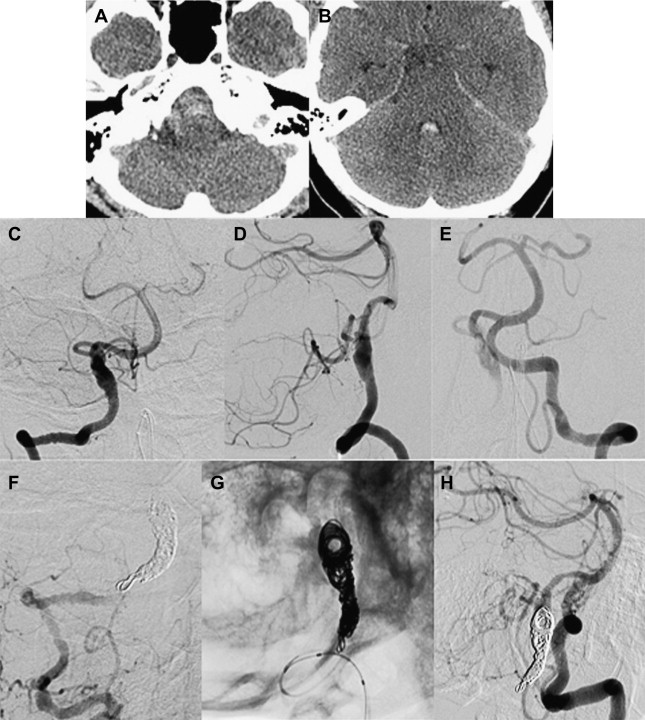
In patients in whom the parent artery can be sacrificed without risking major infarction, endovascular and surgical occlusion of a fusiform aneurysm (and thus the parent artery), is straightforward. For example, fusiform dissecting aneurysms of the intracranial VA below the ipsilateral posterior inferior cerebellar artery (PICA) origin can be successfully treated with detachable coil occlusion of the VA (and, therefore the aneurysm) below the PICA origin ( Fig. 3 ). This technique can be used when the ipsilateral VA anastomoses with the basilar artery, and thus can retrogradely supply the PICA on the side of VA sacrifice. For a fusiform dissecting VA aneurysm involving the dominant VA in which the other VA is diminutive or does not anastomose with the basilar artery, stent coiling the aneurysm to preserve patency of the parent artery is the preferred approach ( Fig. 4 ).
Large dolichoectatic fusiform aneurysms are often difficult to treat by endovascular and surgical techniques if preservation of patency of the parent vessel is essential. It is difficult to place coils circumferentially around a stent placed in a fusiform aneurysm, as visualization of coil placement becomes increasingly challenging after several coils have already been placed. Recently, the concept of placing multiple overlapping stents or stents with a large number of struts (eg, Pipeline stent, Chestnut Medical, Menlo Park, CA, USA) to form a flow diverter and cause thrombosis in fusiform aneurysms but maintain a patent central lumen has become an area of great interest. In these patients, flow diversion may cause enough stasis in an aneurysm to cause thrombosis of the aneurysm even without placement of coils. Risks of occluding small perforating arteries arising from the aneurysm, as well as risk of aneurysm rupture, remain important open questions. An additional downside of such stent-based therapies is the need for dual antiplatelet medication. Bypass trapping is the primary surgical technique for the treatment of fusiform aneurysms in which distal flow must be preserved. In this technically demanding method, clips are placed proximal and distal to the aneurysmal segment (trapping) and a bypass graft (superficial temporal artery, radial artery transposition, or venous transposition) is placed distal to the trapped aneurysm.
Liquid embolic therapy for brain aneurysms has long been an area of interest, and the FDA has recently approved of one agent, and there has been research in others. Onyx HD500 (EV3, Irvine, CA, USA), a more viscous version of the liquid embolic already widely used in preoperative embolization of brain AVMs, has been approved in the United States for humanitarian use in the treatment of intracranial, saccular, sidewall aneurysms that present with a wide neck (4 mm or >) or with a dome/neck ratio less than 2 that are not amenable to treatment with surgical clipping. Aneurysm treatment with a liquid embolic entails placing an embolization catheter in the aneurysm and temporarily inflating a balloon in the parent artery to prevent escape of embolic materials during intraaneurysmal injection. Because many embolic agents face the parent artery in an aneurysm with a broad neck, antiplatelet agents are used in a similar fashion for stent coiling and flow diversion techniques. Liquid embolics can be used either alone or in combination with coils and stents. The most widespread use of Onyx HD500 is not in acutely ruptured aneurysms, but instead in the treatment of aneurysms that have partially recanalized after prior coiling, in essence using the liquid embolic as a caulking agent between the in situ coils. Neucrylate (Valor Medical, San Diego, CA, USA) is another liquid embolic agent currently undergoing preclinical evaluation in the United States and clinical testing overseas. It may ultimately have advantages over other agents because of its faster delivery into aneurysms, which is of particular importance in critically ill patients with SAH. Long-term data on the durability of liquid embolics in the treatment of aneurysms are lacking; until those data exist, coils and coils in combination with adjunctive devices are likely to remain the standard of endovascular care.
Patient demographics and clinical status also play a role in determining optimal aneurysm therapy. The International Study of Unruptured Intracranial Aneurysms (ISUIA) trial comparing clipping with coiling of unruptured aneurysms suggested that patients older than 50 years had poorer surgical outcomes. Patients with higher-grade SAH (Hunt and Hess grades IV and V) and comorbidities such as cardiac stun syndrome are often preferentially coiled because of their tenuous clinical status. Endovascular aneurysm treatment has only existed since the 1970s, and coiling since the early 1990s has been limited by a lack of long-term follow-up. This is not the case for surgical clipping, which has been performed since the 1930s. Thus, for very young patients, a discussion of the relative advantages of endovascular versus surgical aneurysm treatment should include a discussion of the known and expected durability of each treatment. Endovascular approaches to pediatric aneurysms have been highly successful, but extension of the ISAT data to this age group should be tempered by the lack of long-term follow-up data. As a parallel to the ISAT data in which second aneurysms were more likely to be the source of recurrent SAH after clipping or coiling of an index aneurysm, we found that 6 of 77 (8%) children who were treated surgically or endovascularly for an index brain aneurysm developed new or enlarging aneurysms warranting treatment ∼5 years after index aneurysm therapy. Considering the long-expected lifetimes of these patients, long-term follow-up imaging surveillance is essential; MR angiography is our preference given its lack of ionizing radiation. In contrast, adult patients with a previous aneurysmal SAH develop new aneurysms at a rate of 2% per year and have a 6-fold increased risk of recurrent SAH over baseline (6:10,000 persons per year in patients with prior SAH vs 1:10,000 persons per year in the general population). Ruptured brain aneurysms should be regarded as a potentially chronic disease, not just a one-time event. Please refer to the article by Nancy Fischbein and Christine Wijman, Hemorrhagic Stroke and Non-traumatic Intracranial Hemorrhage , in this publication, for additional information about the imaging diagnosis of SAH.
Cerebral hemorrhagic disease
Aneurysmal SAH
Aneurysmal SAH afflicts about 30,000 people annually in the United States, thus constituting about 5% of all strokes and about 30% of all hemorrhagic strokes. Aneurysmal SAH constitutes 80% of cases of nontraumatic SAH, with the remaining 20% divided between perimesencephalic hemorrhage, arteriovenous fistulas (dural and pial), arteriovenous malformations (AVMs), and cavernous malformations that come to the surface of the brain. Perimesencephalic SAH is believed to be caused by a ruptured vein, and thus angiographically occult, and should therefore be a diagnosis of exclusion. Approximately half of patients with aneurysmal SAH die because of the rupture, with most of these deaths occurring within the first 2 weeks after initial hemorrhage. Mortality is front-loaded: 10% die before arrival at the hospital and 25% die within the first 24 hours after aneurysm rupture.
Ruptured brain aneurysms are highly unstable and prone to rerupture. There is at least a 4% rerupture rate within the first 24 hours, and a 30% rerupture rate within the first month. Dissecting aneurysms of the intracranial VA are even more friable and prone to rerupture than other intracranial aneurysms, with rebleeding rates within the first 24 hours exceeding 40% in some case series. Rebleeding of a brain aneurysm carries up to 70% risk of death. In ruptured aneurysms left untreated for long periods of time, risk of rerupture has been reported to decline to about 3% per year, although such natural history studies are few in an era of safe and effective surgical and endovascular aneurysm therapy.
Beyond being life-threatening, aneurysmal SAH also causes long-term disability in many survivors. Between one-third and one-half of SAH survivors have chronic cognitive deficits and concomitantly reduced quality of life. Focal neurologic deficits are less common, as is chronic hydrocephalus requiring ventriculoperitoneal shunting.
The International Subarachnoid Aneurysm Trial (ISAT) of 2143 patients with aneurysmal SAH reported that endovascular coiling of ruptured brain aneurysms geometrically amenable to either coiling or surgical clipping resulted in a lower rate of death or dependency at 1 year after treatment (23.7% for coiling and 30.4% for clipping) such that the trial was halted early because of the superiority of coiling over clipping. More recent long-term follow-up of patients from ISAT (6–14 years, mean 9 years follow-up) confirmed that 5-year survival was superior for patients receiving endovascular coiling versus surgical clipping (14% vs 11%, respectively), but that dependency rates were similar (83% independent vs 82% independent, respectively). There was an overall low (but higher) rate of recurrent SAH after 1 year for the treated aneurysm in coiled patients as opposed to clipped patients (10 vs 3, respectively). 40% of new SAH (11 of 24) were from previously untreated second aneurysms seen at the time of the initial (index) aneurysm treatment, or from entirely new aneurysms not previously detected. Based on ISAT and other data, endovascular therapy has become the treatment of choice for an increasing number of brain aneurysms in the past decade. A recent study of US hospitalizations for treatment of ruptured and unruptured intracranial aneurysms has also concluded that clipping, compared with coiling, was associated with significantly longer lengths of hospitalization and significantly higher total hospital charges for patients with both ruptured and unruptured aneurysm.
Several factors are ideally taken into account in the selection of endovascular versus surgical treatment of any given ruptured aneurysm:
- 1.
Location of the aneurysm
- 2.
Geometry of the aneurysm
- 3.
Age of the patient
- 4.
SAH clinical grade
- 5.
Patient comorbidities
- 6.
Experience of the interventionalist and surgeon
- 7.
Preferences of the patient or patient’s family.
In general, the deeper the location of the aneurysm and more brain retraction that is required to reach it surgically, the more likely that endovascular therapy offers treatment with lower risk of side effects.
Aneurysm geometry is generally defined as narrow-necked saccular, broad-necked saccular, or fusiform. Aneurysms with narrow necks that tend to self-retain coils within the aneurysm sac often are also favorable for straightforward surgical clipping. An additional aspect of aneurysm geometry that should be taken into account is overall size of the aneurysm. Aneurysms less than 2 mm in diameter can be challenging to coil because of their small size, given that the smallest detachable aneurysm coils are 1.5 mm to 2 mm in diameter. However, in a large series of aneurysms 3 mm or less in diameter receiving endovascular coiling, procedural success and complication rates were similar to coiling of larger aneurysms, with a slightly higher procedural rupture rate (mostly asymptomatic) and lower rate (5%) of need for subsequent retreatment. Giant aneurysms (25 mm or greater in diameter), which can be difficult to treat surgically because of their large size, can also be challenging to treat with primary coiling, as they often require many coils to achieve adequate aneurysm occlusion. Endovascular therapy with stents, flow diverters, or liquid embolics (see later discussion) may offer advantages over coiling alone, including a potential reduction in the overall cost of therapy.
Saccular aneurysms with broad necks often require adjuncts for successful endovascular therapy. Balloon-assisted coiling involves temporary inflation of a balloon in the parent artery to encourage coils to assume a self-sustainable stable configuration within the aneurysm. Patients with broad-necked coiled aneurysms are often placed on aspirin following the procedure to reduce parent artery embolic phenomena derived from clots forming on the large coil mass facing the parent artery. Stent-supported coiling consists of positioning a stent in the parent artery across the aneurysm neck to hold coils separately placed in the aneurysm (either through the stent or around the stent). The disadvantage of stent placement for treatment of acutely ruptured aneurysms is the requirement for dual antiplatelet therapy, including both aspirin and clopidogrel, to reduce the risk of stent thrombosis and distal thromboembolization. Although aspirin is generally well tolerated, clopidogrel may increase the risk for secondary hemorrhage during external ventricular drain (EVD) placement. However, at least one center has reported a large series of ruptured aneurysms treated with primary stent coiling without noting any increased frequency of secondary hemorrhage. Early EVD placement to relieve hydrocephalus before surgical or endovascular aneurysm occlusion is prudent for the relief of increased intracranial pressure (ICP) and has a secondary benefit of allowing potentially safer use of clopidogrel or gpIIb/IIIa inhibitors (such as abciximab or eptafibitide) in patients in whom a stent is necessary to achieve stable coil position.
In patients in whom the parent artery can be sacrificed without risking major infarction, endovascular and surgical occlusion of a fusiform aneurysm (and thus the parent artery), is straightforward. For example, fusiform dissecting aneurysms of the intracranial VA below the ipsilateral posterior inferior cerebellar artery (PICA) origin can be successfully treated with detachable coil occlusion of the VA (and, therefore the aneurysm) below the PICA origin ( Fig. 3 ). This technique can be used when the ipsilateral VA anastomoses with the basilar artery, and thus can retrogradely supply the PICA on the side of VA sacrifice. For a fusiform dissecting VA aneurysm involving the dominant VA in which the other VA is diminutive or does not anastomose with the basilar artery, stent coiling the aneurysm to preserve patency of the parent artery is the preferred approach ( Fig. 4 ).