CHAPTER 97 Acute Pulmonary Thromboembolic Disease
Pulmonary embolism (PE) was clinically described in the early 1800s, and von Virchow first described the connection between venous thrombosis and PE.1,2 In 1922, Wharton and Pierson reported the first radiographic description of PE,3 a commonly encountered entity whose diagnosis is clinically challenging because of its nonspecific symptoms.
Prevalence and Epidemiology
PE is a potentially fatal condition with substantial morbidity and mortality in untreated patients. The annual incidence of pulmonary embolism is estimated to be between more than 300,000 cases, resulting in approximately 50,000 to 100,000 deaths in the United States every year.4 Nearly 90% of PEs arise from lower extremity deep venous thrombosis (DVT). Diagnosis of pulmonary embolism remains a clinical challenge because of its nonspecific presentation. Mortality ranges from 3.5% to 15% and can be as high as 31% to 58% in the presence of shock.5,6 Hence, prompt and accurate diagnosis is imperative because 65% of patients die within 1 hour of presentation and approximately 93% in the first 2.5 hours.7 Since its original description, imaging has played an important role in the diagnosis of PE. For many years, ventilation-perfusion (V/Q) scintigraphy has been the main imaging modality for the evaluation of patients with suspected PE. However, with the advent of and the widespread availability of faster computed tomography (CT) scanners, CT scanning has emerged as another important diagnostic test for the evaluation of not only PE but also DVT in select patients. Thus, familiarization with various aspects of imaging evaluation is of extreme importance for the interpreting physician.
Etiology and Pathophysiology
Three primary influences predispose a patient to thrombus formation, which form the so-called Virchow triad: (1) endothelial injury; (2) stasis or turbulence of blood flow; and (3) blood hypercoagulability.1,2,8
More than 90% of all PEs arise from thrombi within the large deep veins of the legs, typically the popliteal vein and the larger veins central to it.1,2,4 The pathophysiologic consequences of thromboembolism in the lung largely depend on the cardiopulmonary status of the patient and on the size of the embolus, which in turn dictates the size of the occluded pulmonary artery.
MANIFESTATIONS
Clinical Presentation and Pretest Probability
Many patients with suspected PE present with nonspecific findings such as acute chest pain, and some are even asymptomatic. Some observers have suggested that because of the decline in tolerance for diagnostic uncertainty, diagnostic studies for the evaluation of PE may be overused in current practice. Pretest probability is an important concept, because the sensitivity, specificity, and predictive values of diagnostic imaging modalities are based on the pretest probability of PE, which is usually determined by clinical findings and laboratory test results.9–11 The Wells criteria (Table 97-1)12 and Geneva score,13 which were devised and validated for clinical pretest probability, have been reported to have similar accuracy levels. Chagnon and colleagues14 have shown that these methods can be used to divide patients into low-, intermediate-, and high-probability groups for PE, although neither was accurate enough to diagnose PE in individual cases.
TABLE 97-1 Wells Clinical Decision Rule for Pulmonary Embolism
| Variable | Points |
|---|---|
| Clinical signs and symptoms of deep vein thrombosis | 3.0 |
| Alternative diagnosis less likely than pulmonary embolism | 3.0 |
| Heart rate > 100/min | 1.5 |
| Immobilization (> 3 days) or surgery in the previous 4 weeks | 1.5 |
| Previous pulmonary embolism or deep vein thrombosis | 1.5 |
| Hemoptysis | 1.0 |
| Malignancy (receiving treatment; treated in the last 6 months or palliative) | 1.0 |
Clinical probability of pulmonary embolism unlikely—4 points or less.
Clinical probability of pulmonary embolism likely—more than 4 points.
Adapted from Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost 2000; 83:416-420.
Serum D-dimer levels can be used to gauge the presence of PE. D-dimer is a fibrin degradation product that increases with clot lysis, suggesting the presence of thrombosis. It has been shown that this test with levels above a threshold value of 0.5 mg/L has an excellent sensitivity of 95%, but the specificity is lower (38% to 83%)15 because of elevated levels in some conditions, such as active inflammation, infection, pregnancy, malignancy, and liver failure. Of all the methods available for measurement of D-dimer levels (Table 97-2),10 the VIDAS D-dimer assay (bioMérieux, Marcy l’Etoile, France), a rapid, quantitative, enzyme-linked immunosorbent assay (ELISA) procedure, has been reported to have a 96.4% sensitivity,16 thus ruling out PE in the absence of a calculation of clinical pretest probability.16,17 However, this is not routine clinical practice. Several studies10,11,18 have demonstrated that a negative result of rapid quantitative ELISA D-dimer can be combined with the clinical probability score to exclude PE in up to 40% of patients, thereby eliminating further workup.
TABLE 97-2 Sensitivity and Specificity (%) of D-Dimer Based on Laboratory Technique
| Laboratory Technique | Mean (Range) | |
|---|---|---|
| Sensitivity | Specificity | |
| ELISA | 95 (85-100) | 44 (34-54) |
| Rapid quantitative ELISA | 95 (83-100) | 39 (28-51) |
| Rapid semiquantitative ELISA | 93 (79-100) | 36 (23-50) |
| Rapid qualitative ELISA | 93 (74-100) | 68 (50-87) |
| Quantitative latex agglutination | 89 (81-98) | 45 (36-53) |
| Semi quantitative latex agglutination | 92 (79-100) | 45 (31-59) |
| Whole blood agglutination | 78 (64-92) | 74 (60-88) |
ELISA, enzyme-linked immunosorbent assay.
Adapted from Ginsberg JS, Wells PS, Kearon C, et al. Sensitivity and specificity of a rapid whole-blood assay for D-dimer in the diagnosis of pulmonary embolism. Ann Intern Med 1998; 129: 1006-1011.
Imaging Techniques and Findings
Chest Radiography
The Westermark sign (Fig. 97-1)19 is a focal peripheral lucency beyond an occluded vessel accompanied by mild dilation of central pulmonary vessels, seen in 7% to 14% of cases of documented PE in the PIOPED study. It is a subtle finding caused by embolic obstruction or hypoxic vasoconstriction of pulmonary artery.
Another finding is enlarged central pulmonary vasculature, which can be easily missed. It may be caused by vessel distention by thrombus or by an acute rise in pulmonary arterial pressure secondary to distal emboli. A sausage-like configuration of focal enlargement of the right descending interlobar pulmonary artery caused by the physical presence of a clot has also been noted in many patients with acute PE and referred to as the knuckle sign. Hampton hump20 is classically referred to as a conical peripheral opacity pointing toward the hilum. These are multiple, subpleural lower lobe infarcts seen as ill-defined opacities without air bronchography (Figs. 97-2 and 97-3).
Focal parenchymal abnormalities are the most commonly encountered lesions on chest radiographs in PIOPED series patients. These particularly include subsegmental atelectasis (Fleischner lines),21 seen as linear opacities in lung bases; these are transient in nature and thought to be caused by mucus plugging, hypoventilation, or distant airway closure. Focal air space consolidation represents true pulmonary infarction, with ischemic necrosis or pulmonary hemorrhage without infarction occurring in 10% to 60% of patients. Small, unilateral, pleural effusions and diaphragmatic elevation are seen in a large number of PE patients.
Lower and Upper Extremity Ultrasonography
As noted, 90% of PEs originate from thrombosis within the deep venous system of the lower extremities and are mostly clinically silent; other sources are the pelvic, renal, and upper extremity veins.22 Significant morbidity and mortality are associated with undiagnosed DVT; thus, methods such as contrast venography (CV), magnetic resonance venography (MRV), and lower extremity ultrasonography are commonly used for its detection. Real-time gray scale, continuous wave and pulsed Doppler, and color Doppler imaging, along with ancillary techniques such as the Valsalva maneuver and manual blood flow augmentation, are used to image the lower extremity veins.
Color Doppler imaging is useful to identify deeper veins such as the iliac veins and superficial femoral veins, for which direct venous compression is not possible, or for obese or swollen extremity patients. Thrombosis is identified as the absence of color flow within a vessel lumen at baseline and with augmentation; the sensitivity and specificity are 95% and 98%, respectively, for femoropopliteal DVT.22
Ventilation-Perfusion Scintigraphy
A normal perfusion scan excludes a diagnosis of PE, with its negative predictive value close to 100%.23–25 It is a safe, relatively inexpensive, and widely available test that allows the acquisition of single-breath, equilibrium, and washout images. A high-probability V/Q scan (Figs. 97-4 and 97-5), in combination with high-probability clinical findings, corresponded to PE in 96% of patients, whereas a low-probability clinical assessment showed PE in only 4% of patients in a major study.9
In PIOPED-1, a V/Q scan in patients with a normal chest radiograph was diagnostic in 52% of patients with suspected PE,26 and one study has shown it to be diagnostic in 91% of patients.27 This PIOPED group retrospectively analyzed perfusion scans alone and found the results to be equivalent to those of the V/Q technique.28 In the PISA-PED trial,29 only perfusion scans were obtained. These were classified according to the shape of defects, yielding sensitivity and specificity of 86% and 93%, respectively, for abnormal perfusion scans compatible with PE, with the result being dichotomous. These studies indicate that the ventilation scan can be eliminated, thus reducing cost and radiation dose. It can also be used as a preferred alternative for patients unable to undergo CT angiography.
It can be used particularly for reproductive female patients whose chest radiograph is likely to be normal and in whom the breast irradiation dose from CT angiography can be minimized using the perfusion scan only.9,30 Another significant use is follow-up of proven PE patients undergoing anticoagulation.
Ventilation-Perfusion Single Photon Emission Computed Tomography
V/Q single photon emission computed tomography (SPECT) is a newer modality that may improve both sensitivity and specificity of the V/Q scan (Fig. 97-6). Reinartz and associates31 analyzed 83 patients who underwent four-slice spiral CT, V/Q planar, and V/Q SPECT studies, giving sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) of 97%, 91%, 98%, and 90%, respectively, for SPECT techniques. This study used aerosolized technetium 99m (Tc 99m) for the ventilation portion of the V/Q scan instead of the radioisotope xenon 133. Tc 99m is five times smaller in diameter and has a 20% efficiency of pulmonary deposition in comparison to 2% for xenon 133, thus helping improve the results.32,33 Unlike PIOPED, this study considered all the mismatched results as PE, regardless of the size. This technique has the potential to improve the interpretation of V/Q scanning by incorporating computerized interpretations.

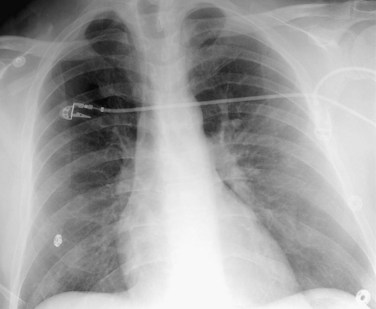
 FIGURE 97-1
FIGURE 97-1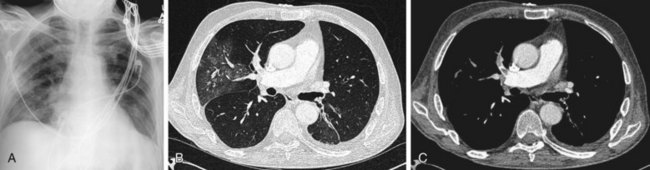
 FIGURE 97-2
FIGURE 97-2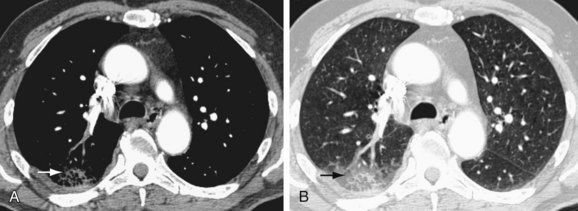
 FIGURE 97-3
FIGURE 97-3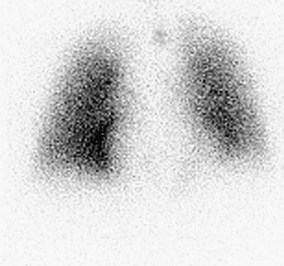
 FIGURE 97-4
FIGURE 97-4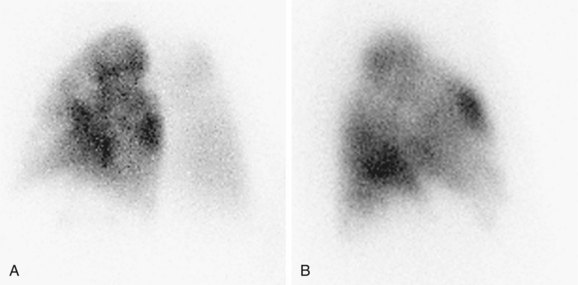
 FIGURE 97-5
FIGURE 97-5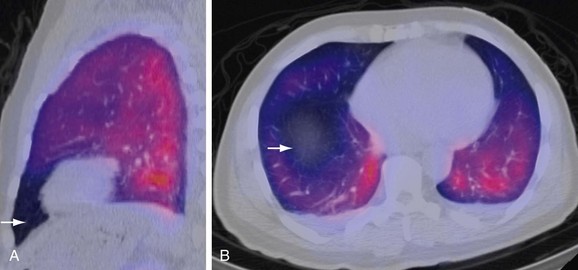
 FIGURE 97-6
FIGURE 97-6

