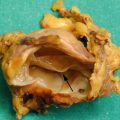
Fig. 1
Ductal adenocarcinoma. (a) Surgical specimen (pancreaticoduodenectomy): pancreatic ductal adenocarcinoma (asterisk) presenting as a white hard mass with irregular margins. Both the Wirsung duct (W) and the common bile duct (C) are dilated. (b, c) Histopathology: the neoplastic tissue is composed by few glandular structures in abundant fibrous desmoplastic stroma. Immunohistochemical staining with CD34 (brown traces in c) reveals scant blood vessels within the lesion (Courtesy of Piccin Editore, Milan, Italy)
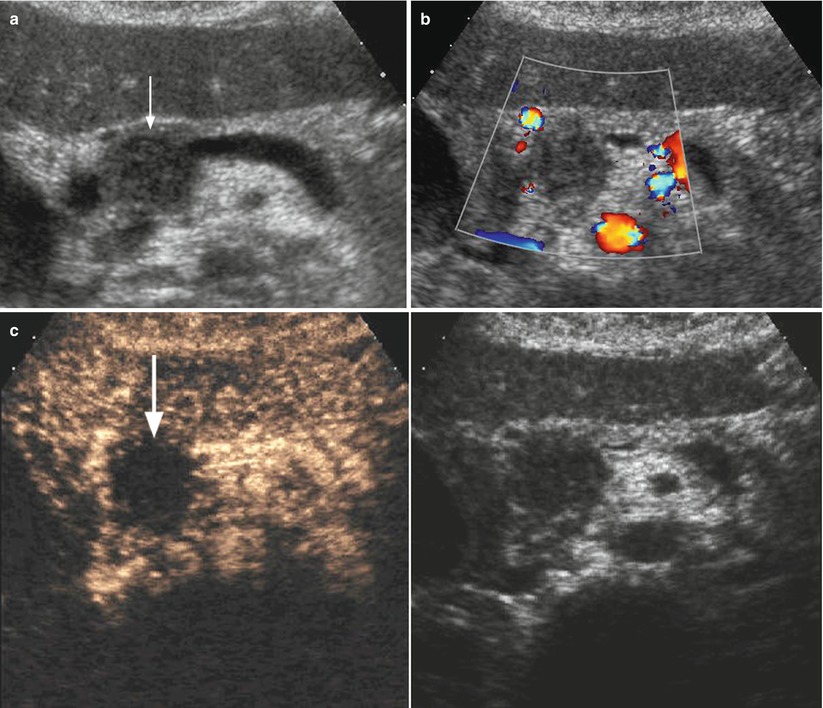
Fig. 2
Ductal adenocarcinoma. (a) Conventional US study: typical US presentation of pancreatic ductal adenocarcinoma as a hypoechoic solid mass (arrow). (b) Color-Doppler analysis: no Doppler signal is visible within the lesion according to the very low vascular density of the neoplastic tissue. (c) CEUS: the lesion typically shows a markedly hypovascular pattern (arrow)
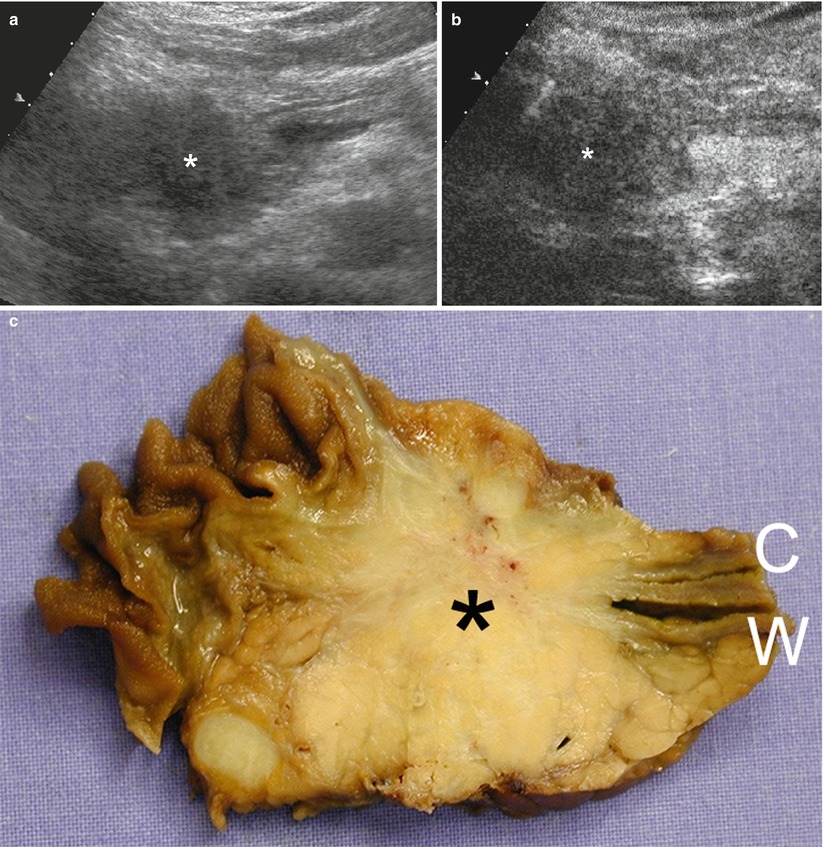
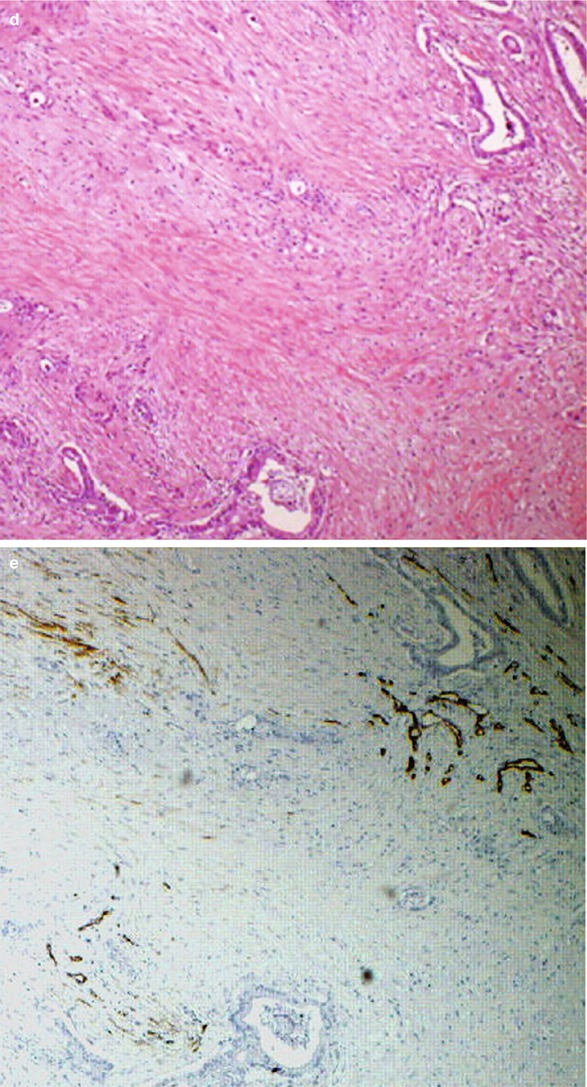
Fig. 3
Ductal adenocarcinoma. (a) Conventional US study: hypoechoic round-shaped lesion (asterisk) with blurred margins located in the pancreatic head, with a slight dilation of the Wirsung duct. (b) CEUS: the lesion typically shows a markedly hypovascular pattern (asterisk). (c) Surgical specimen (pancreaticoduodenectomy): pancreatic ductal adenocarcinoma of the pancreatic head presenting as a white stiff highly fibrotic mass with irregular margins infiltrating the Wirsung duct (W) and the common bile duct (C). (d, e) Histopathology: the neoplastic tissue is typically composed by few glandular structures in abundant fibrous desmoplastic stroma. Immunohistochemical staining with CD34 (brown traces in e) reveals scant blood vessels within the lesion
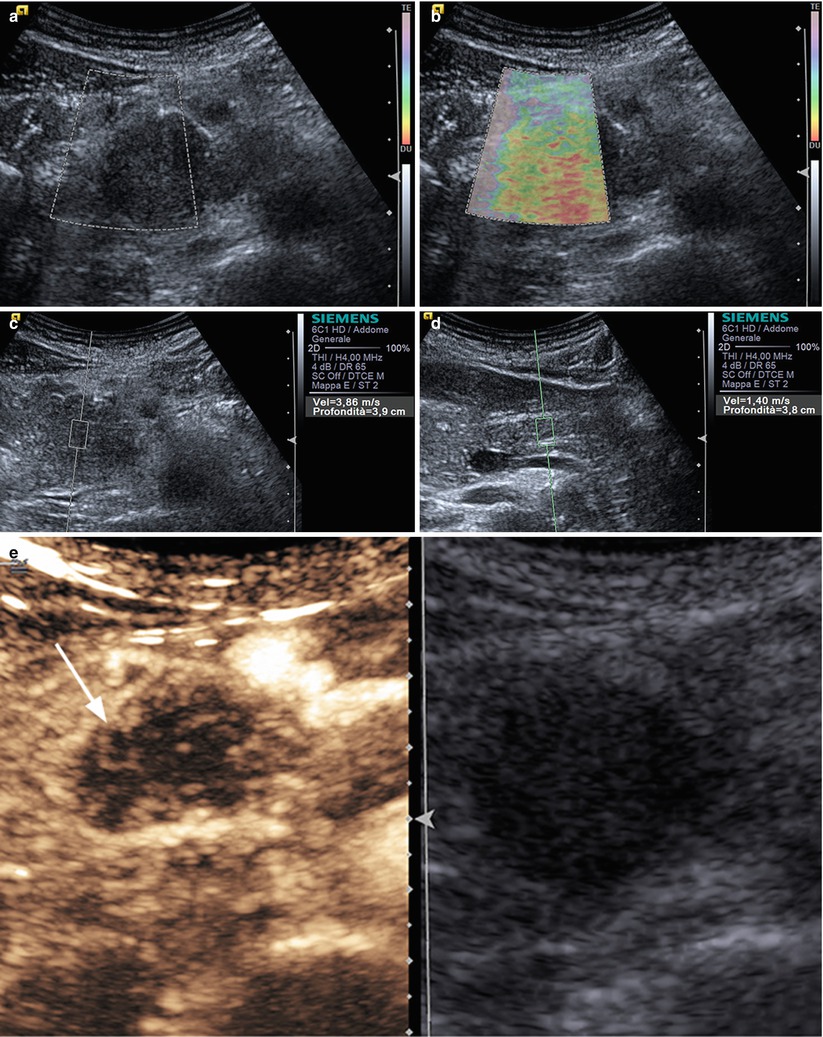
Fig. 4
Ductal adenocarcinoma. (a–e) Complete US study: round-shaped hypoechoic mass (box in a) located in the pancreatic head, resulting stiff, (red) due to the rich desmoplastic stroma in the elastogram (b). At the quantitative ARFI elastography, high-velocity value (3,86 m/s in c) is confirmed in respect to the pancreatic body parenchyma (1,40 m/s in d). At CEUS (e), the lesion typically shows a markedly hypovascular pattern (arrow in e)
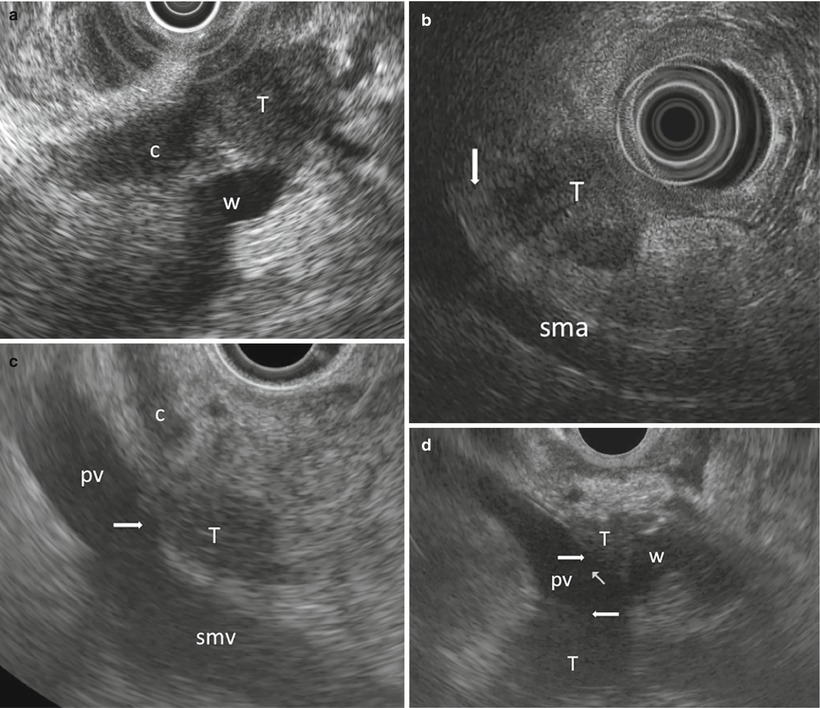
Fig. 5
Ductal adenocarcinoma. (a) EUS study: small tumor (T) obstructing both the common bile duct (c) and the Wirsung duct (w). (b) EUS study: staging of a pancreatic tumor (T) shows a clear interface (arrow) with the superior mesenteric artery (sma). (c) EUS study: staging of a pancreatic tumor (T) shows the contact (arrow) between the lesion and the superior mesenteric vein (smv) and portal vein (pv) wall; c, common bile duct. (d) EUS study: staging of a pancreatic tumor (T) shows the loss of interface (arrows) between the lesion and the portal vein (pv), which is surrounded by the tumor; the Wirsung (w) is obstructed by the tumor
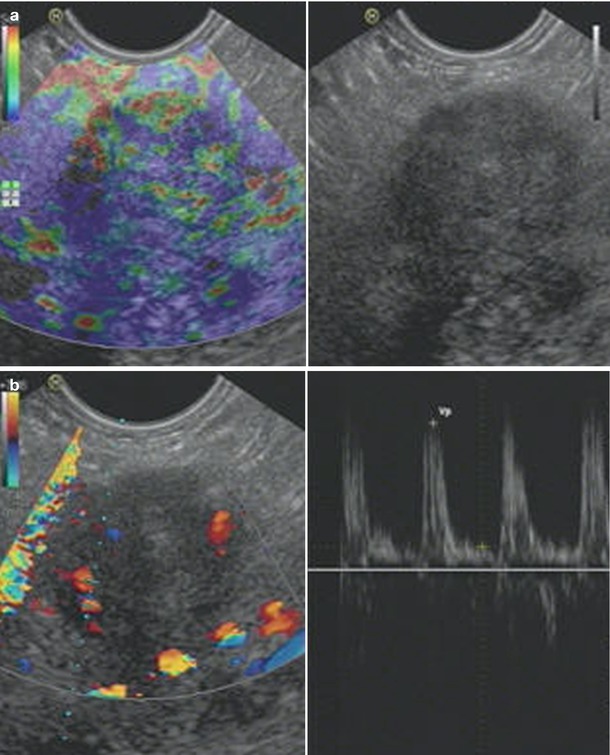
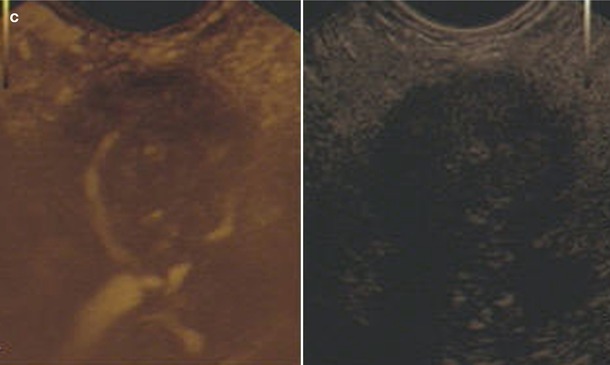
Fig. 6
Ductal adenocarcinoma. (a–c) EUS study: elastography (a), contrast-enhanced endoscopic ultrasound in high MI Doppler mode with microvessel analysis (b) and contrast-enhanced endoscopic ultrasound in low MI mode (c). Stiff structure (blue) in elastography; only a few arterial vessels are visible using pulse-waved Doppler mode, and markedly low contrast-enhancing effect in low MI mode
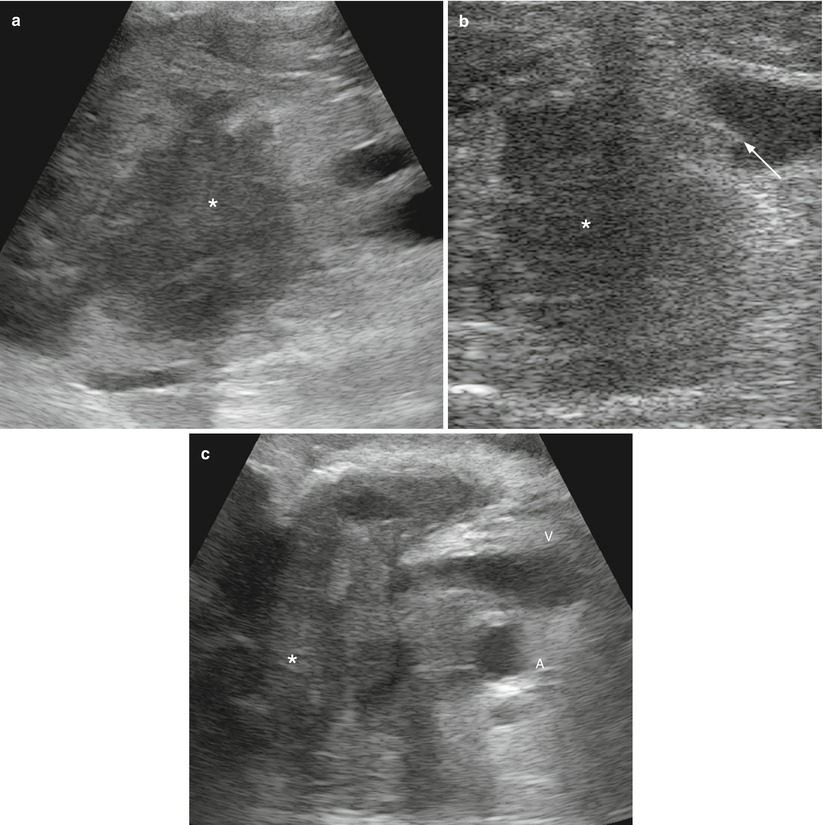
Fig. 7
Ductal adenocarcinoma. (a) IOUS: pancreatic head mass (asterisk) not involving the main vessels. (b) IOUS: pancreatic head hypoechoic mass (asterisk) not separated from the superior mesenteric vein with a small neoplastic portion (arrow) within the lumen of the vein. The wall of the vein appears irregular with deformation of the vessel. (c) IOUS: pancreatic head mass (asterisk) involving the superior mesenteric vein (V) and artery (A)
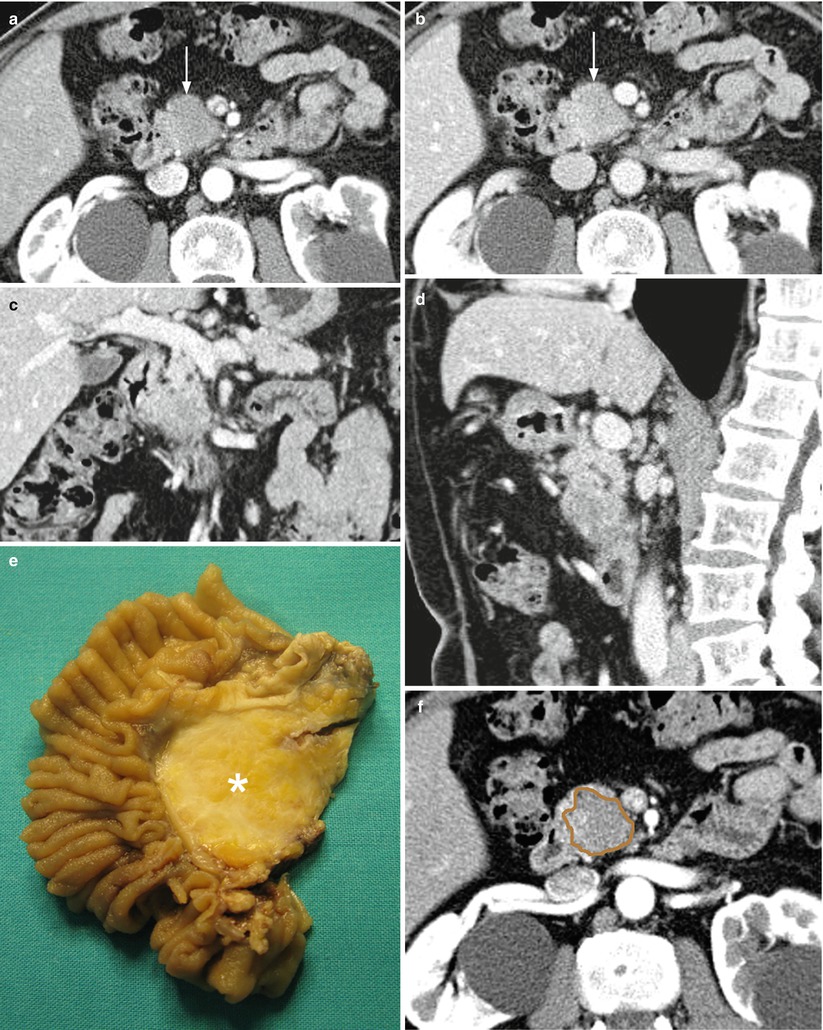
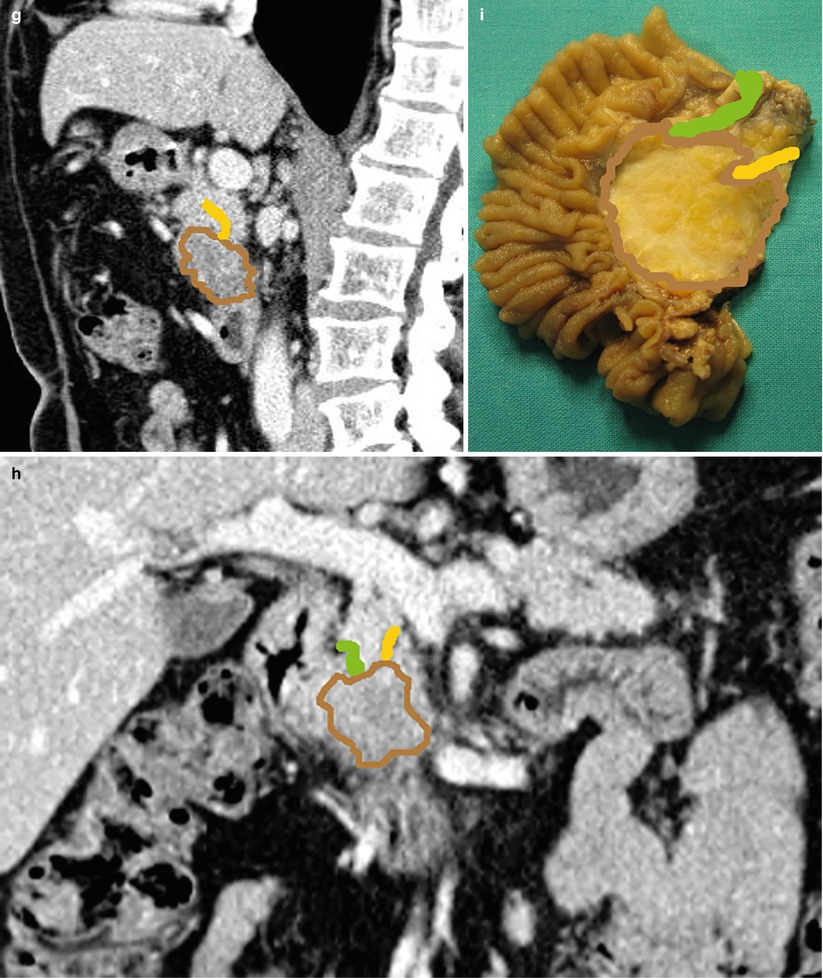
Fig. 8
Ductal adenocarcinoma. (a–d) dynamic CT: the study demonstrates a round-shaped lesion, hypovascular hypodense (arrow) in the pancreatic (a) and venous (b) phases with blurred margins located in the pancreatic head. Coronal (c) and sagittal (d) planes show the abrupt cutoff of the Wirsung duct, which appears slightly upstream-dilated, and of the common bile duct. The superior mesenteric vessels are normal. (e) Surgical specimen (pancreaticoduodenectomy): the presence of a round-shaped ductal adenocarcinoma (asterisk) of the pancreatic head is confirmed. (f–i) Superimposed drawings on CT images (f–h) and specimen (i) better clarify the relationships among the lesion (brown circle), the Wirsung duct (yellow line), and the common bile duct (green line)
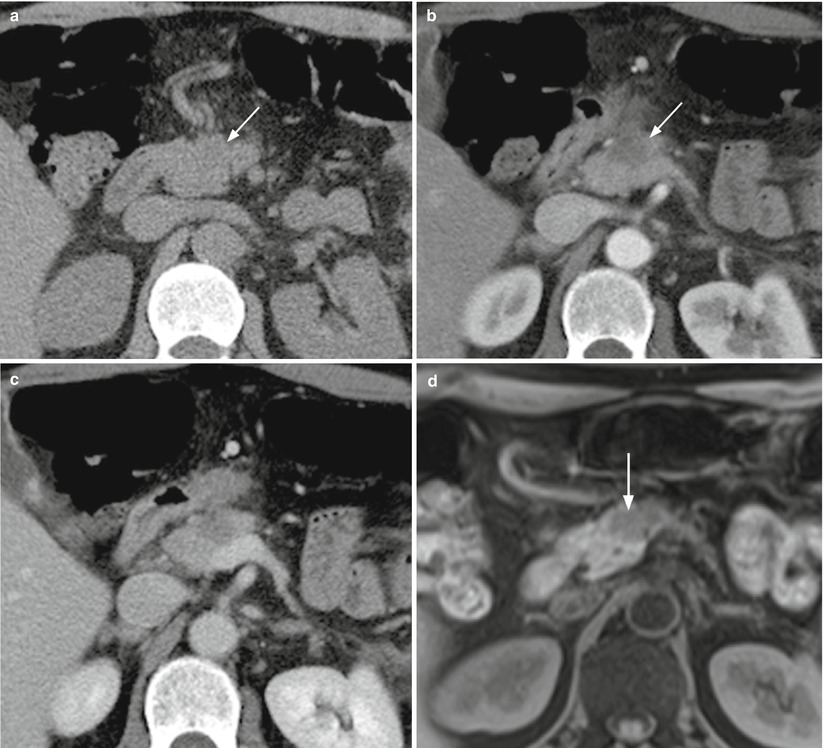
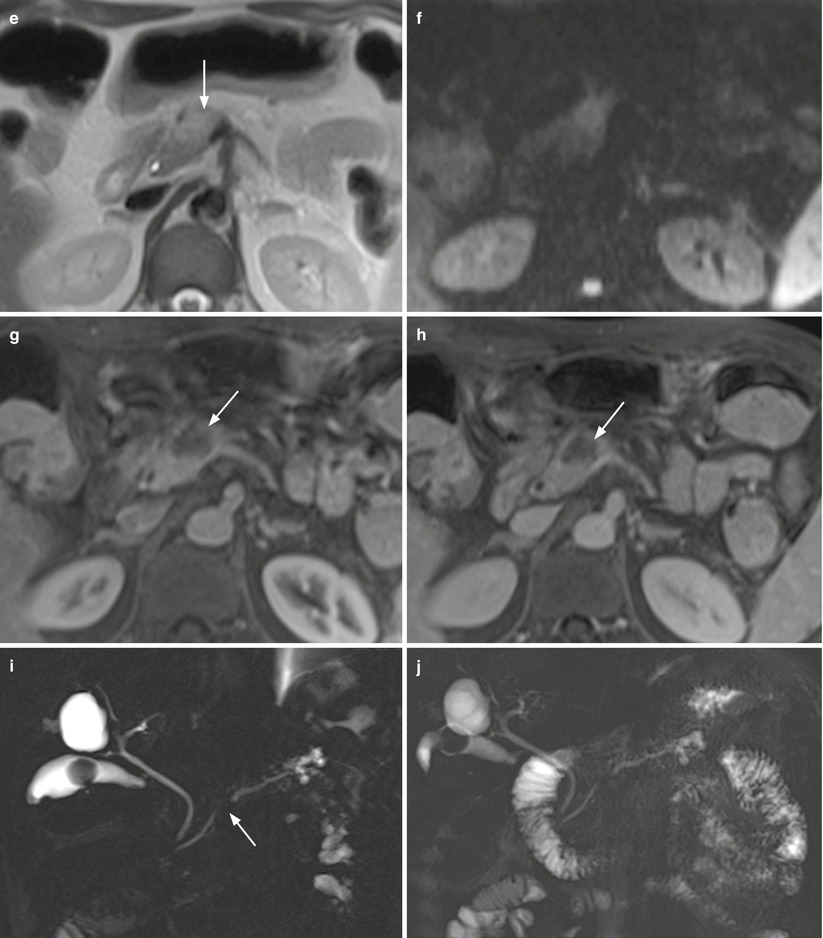
Fig. 9
Ductal adenocarcinoma imaging correlation. (a–c) CT study: typical appearance of a small pancreatic ductal adenocarcinoma, presenting as slightly hypodense (arrow in a) lesion in the baseline (a) acquisition with blurred margins in the pancreatic head, typically hypovascular (arrow in b) in comparison to the adjacent pancreatic parenchyma in the pancreatic (b) and venous (c) dynamic phases. (d–h) MRI study: the lesion typically appears hypointense (arrow in d) on T1-weighted fat-saturated images and slightly hyperintense (arrow in e) on T2-weighted images, with diffusion restriction, appearing hyperintense on high b value DW images (f). At dynamic study, the lesion appears hypovascular (arrow in g and h) in comparison to the adjacent pancreatic parenchyma in the pancreatic (g) and venous (h) dynamic phases. (i, j) MRCP: the neoplasm causes an abrupt cut-off of the Wirsung duct (arrow in i), upstream-dilated, not regressing after secretin stimulation (j)
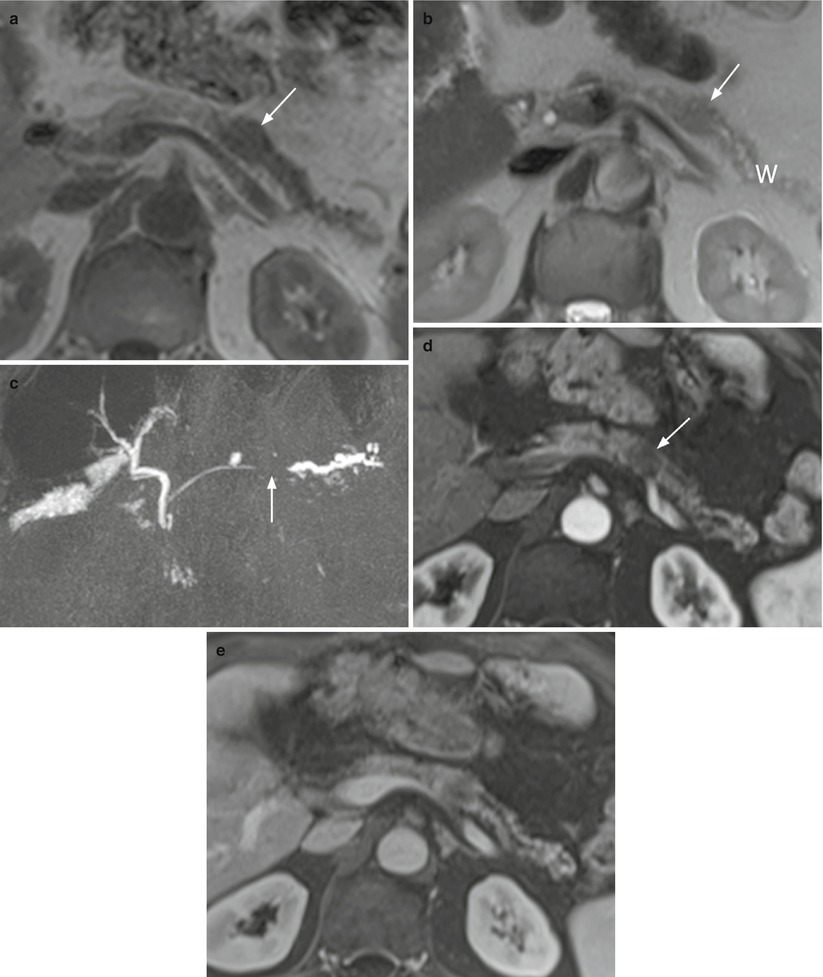
Fig. 10
Ductal adenocarcinoma. (a–e) MRI study: small oval-shaped nodule of the pancreatic body appearing hypointense (arrow in a) on T1-weighted images and slightly hypointense (arrow in b) on T2-weighted images. The upstream portion of the Wirsung duct (W in b) is dilated with atrophic pancreatic parenchyma. MRCP study better shows the abrupt cut-off (arrow in c) and the upstream dilation of the Wirsung duct. At dynamic study, the lesion appears typically hypovascular (arrow in d) in comparison to the adjacent pancreatic parenchyma in the pancreatic (d) and venous (e) dynamic phases
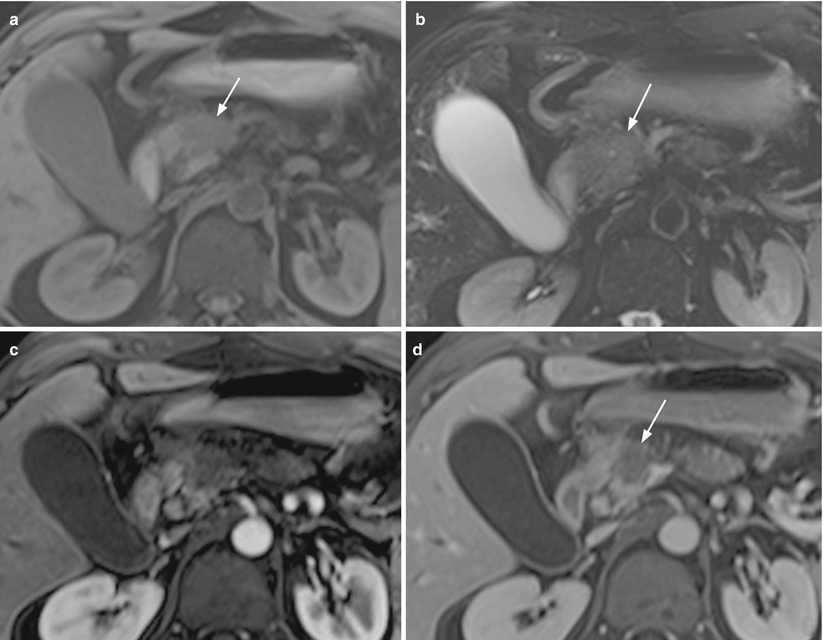
Fig. 11
Ductal adenocarcinoma. (a–d) MRI study: small irregular-shaped lesion of the pancreatic head appearing hypointense on T1- weighted (arrow in a) and slightly hypointense on T2-weighted (arrow in b) fat-saturated images. At dynamic study, the lesion appears typically hypovascular (arrow in d) in comparison to the adjacent pancreatic parenchyma in the pancreatic (c) and venous (d) dynamic phases
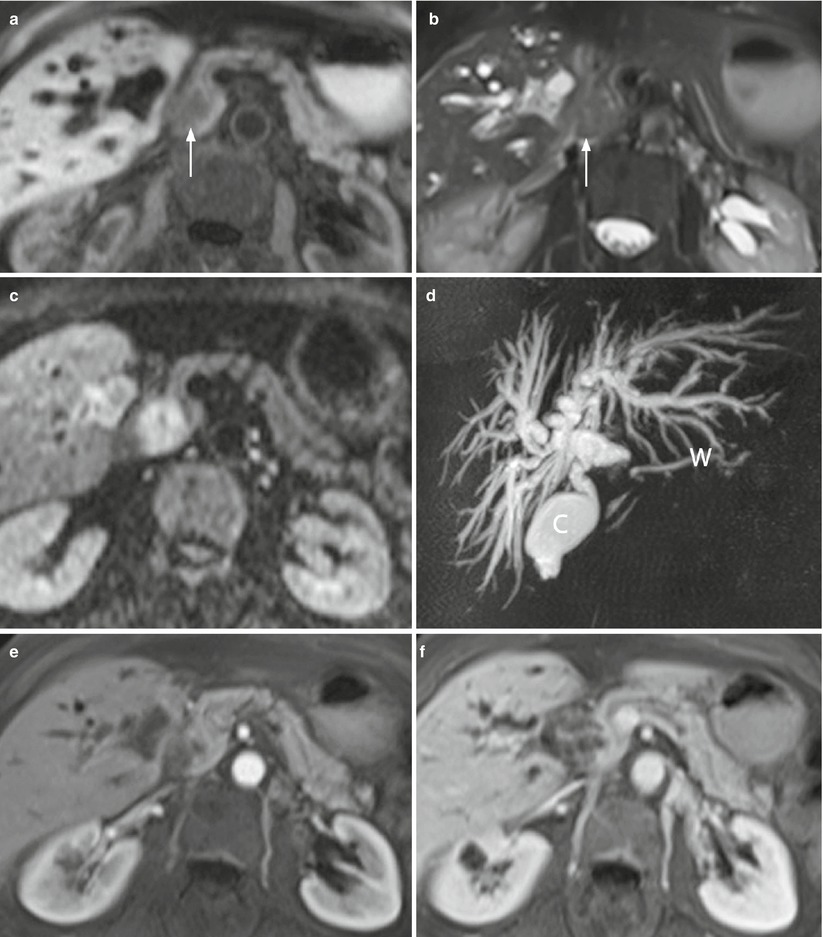
Fig. 12
Ductal adenocarcinoma. (a–f) MRI study: small round-shaped lesion with blurred margins of the pancreatic head, appearing hypointense (arrow in a) on T1-weighted fat-saturated images and slightly hyperintense (arrow in b) on T2-weighted fat-saturated images, and with diffusion coefficient restriction with high b value on DWI (c). MRCP shows a markedly dilated biliary tree, with an abrupt cut-off of the common bile duct (C in d) in the head of the pancreas and a dilated Wirsung duct (W in d). At dynamic study (e, f), the lesion appears almost isovascular in comparison to the adjacent pancreatic parenchyma
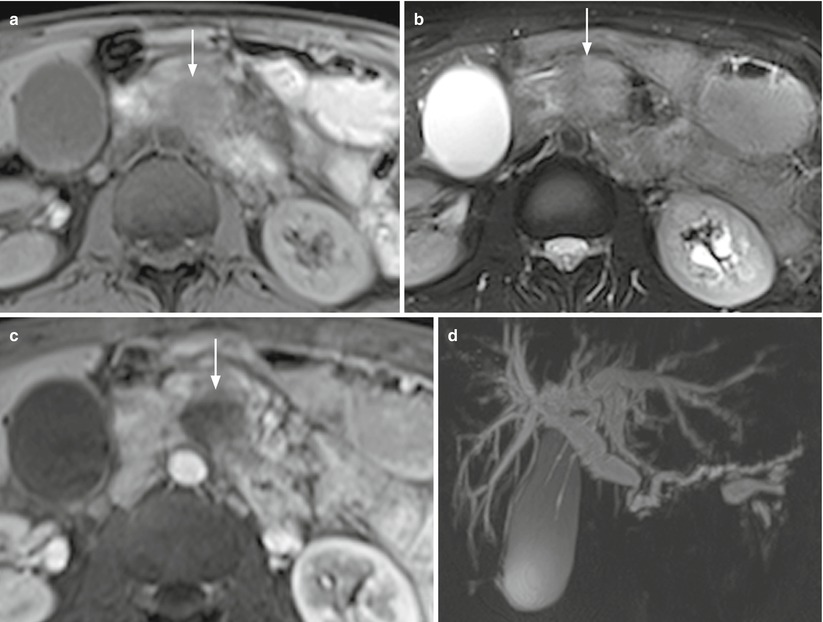
Fig. 13
Ductal adenocarcinoma with double-duct sign. (a–d) MRI study: pancreatic head-neck round-shaped mass with blurred margins, typically appearing hypointense (arrow in a) on T1-weighted fat-saturated images and slightly hyperintense (arrow in b) on T2-weighted fat-saturated images. At dynamic study, the lesion appears markedly hypovascular (arrow in c) in comparison to the adjacent pancreatic parenchyma in the postcontrastographic pancreatic phase. MRCP (d) shows a significant dilation of the biliary tree and Wirsung duct (double-duct sign)
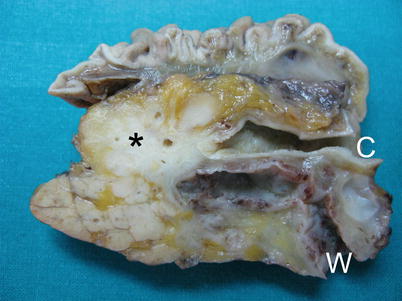
Fig. 14
Ductal adenocarcinoma with double-duct sign. Surgical specimen (pancreaticoduodenectomy): typical macroscopic appearance of pancreatic ductal adenocarcinoma, presenting as a hard, whitish lesion (asterisk) with irregular faint margins. Tumoral margins present infiltrative growth pattern, with the involvement of Wirsung duct (W) and common bile duct (C), both upstream-dilated (double-duct sign)
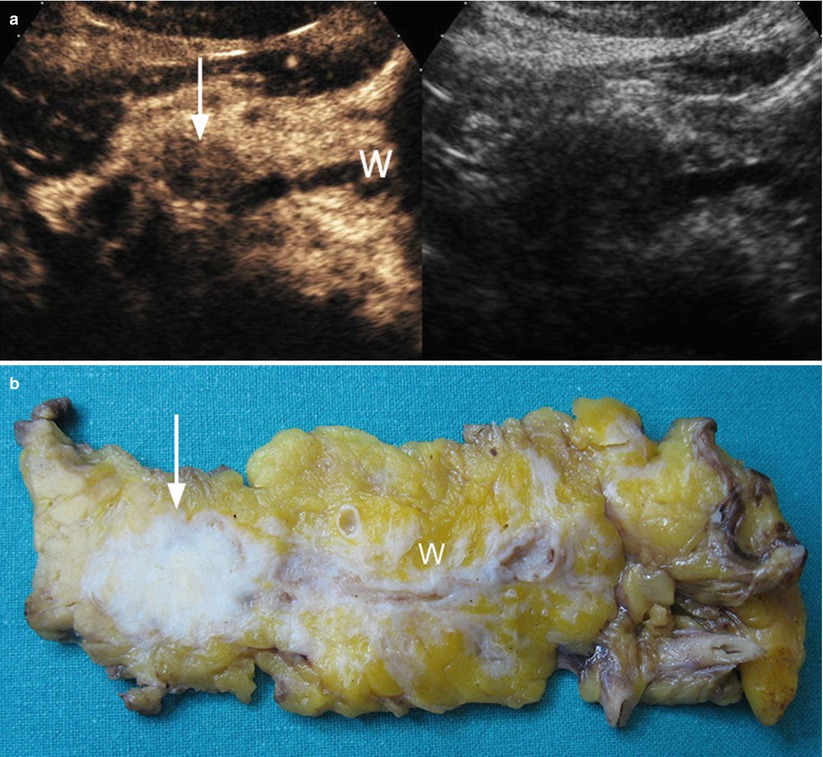
Fig. 15
Ductal adenocarcinoma with main pancreatic duct dilation. (a) CEUS: the study shows a hypoechoic hypovascular round-shaped lesion (arrow) of the pancreatic body, causing upstream dilation of the Wirsung duct (W). (b) Surgical specimen (distal pancreatectomy) of different case: hard, whitish round-shaped ductal adenocarcinoma (arrow) in the pancreatic body with a slightly dilated Wirsung duct (W)
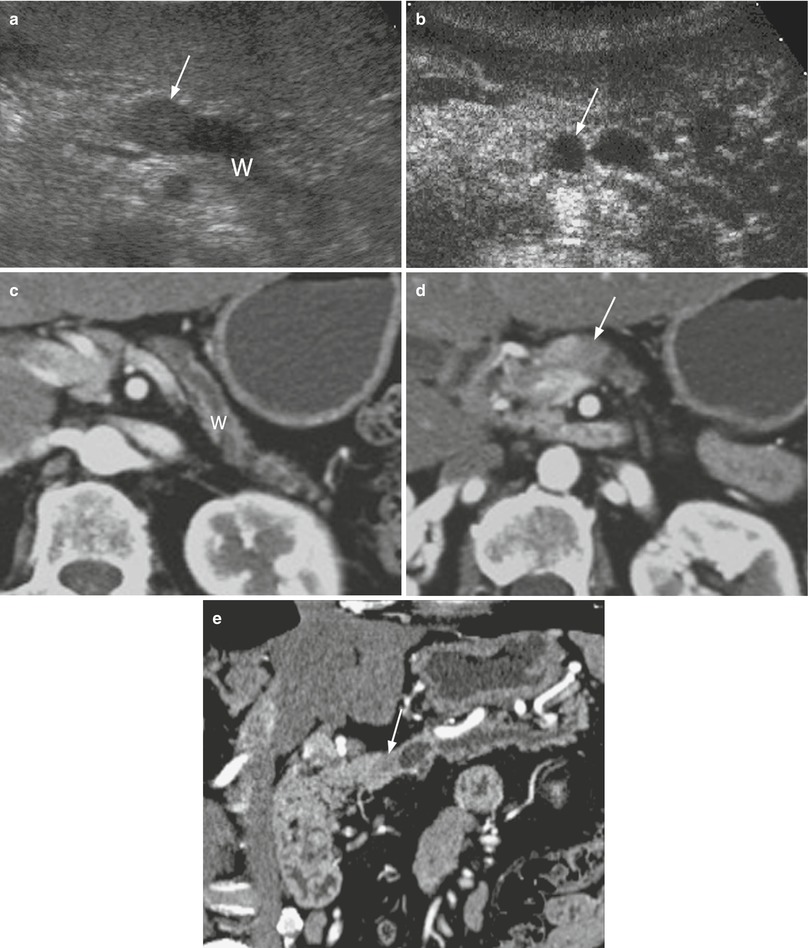
Fig. 16
Ductal adenocarcinoma with main pancreatic duct dilation. (a, b) US study: small round-shaped lesion, hypoechoic (arrow in a) at conventional ultrasound and hypovascular (arrow in b) at CEUS in the pancreatic neck, with marked upstream dilation of the Wirsung duct (W in a). (c–e) dynamic CT: dilated Wirsung duct (W in c), stopped in hypovascular hypodense nodule (arrow in d). Multiplanar reconstruction (e) with MinIP algorithm clearly demonstrates the small hypovascular nodule (arrow in e) in the pancreatic neck, causing upstream dilation of the Wirsung duct
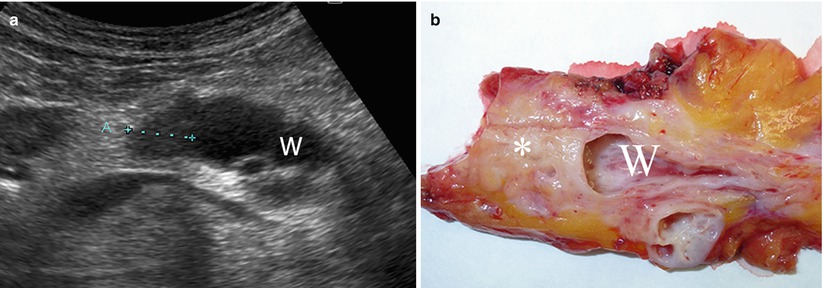
Fig. 17
Ductal adenocarcinoma with main pancreatic duct dilation. (a) Ultrasound study: small round-shaped hypoechoic nodule (calipers) in the pancreatic body causing marked upstream dilation of the Wirsung duct (W). (b) Surgical specimen (distal pancreatectomy): small ductal adenocarcinoma of pancreatic body (asterisk) with markedly dilated Wirsung duct (W)
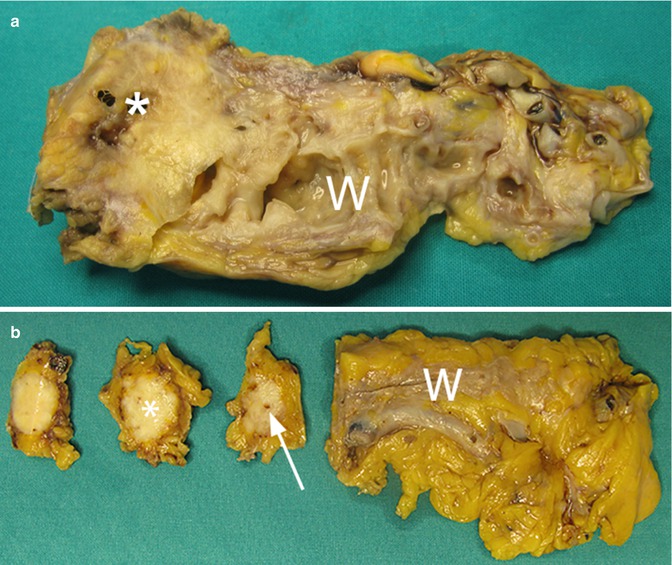
Fig. 18
Ductal adenocarcinoma with main pancreatic duct involvement. (a) Surgical specimen (distal pancreatectomy): pancreatic ductal adenocarcinoma (asterisk), causing marked upstream dilation of the Wirsung duct (W). (b) Surgical specimen of different case: small ductal adenocarcinoma (asterisk) with stenosis (arrow) of the Wirsung duct (W), not upstream-dilated
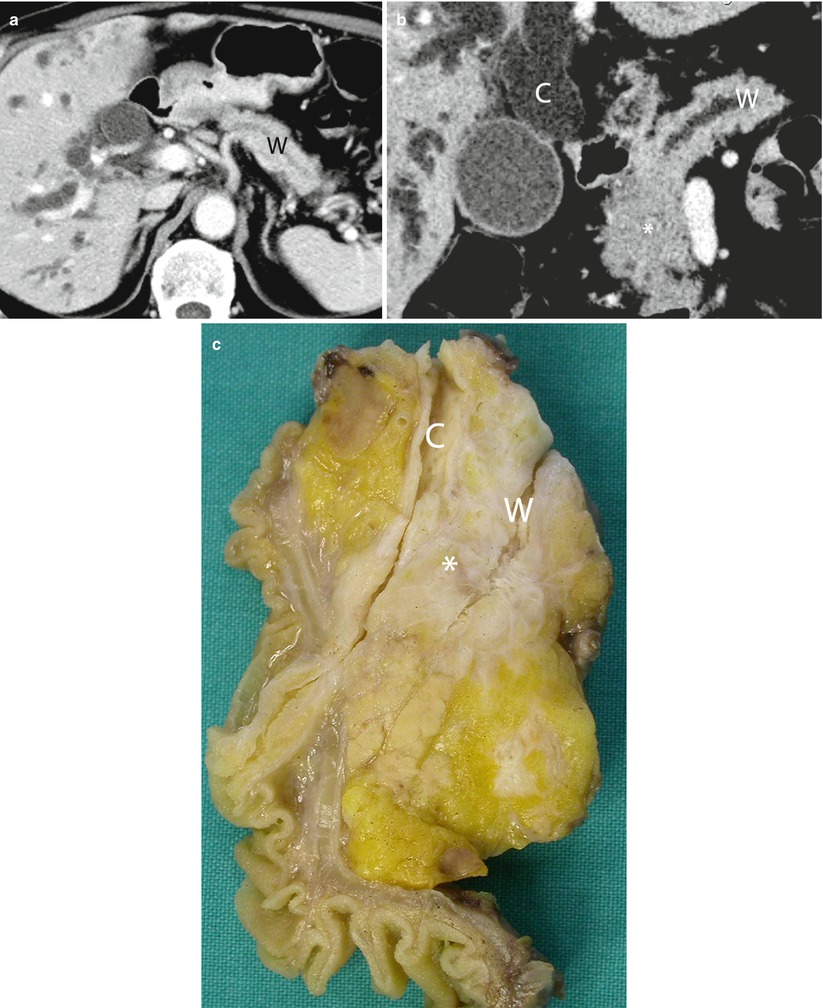
Fig. 19
Double-duct sign. (a, b) dynamic CT: the study shows dilation of the biliary tree and of the Wirsung duct (W in a). Multiplanar reconstruction with MinIP algorithm shows a hypodense mass (asterisk in b) in the pancreatic head, causing dilation of both the common bile duct (C in b) and the Wirsung duct (W in b). (c) Surgical specimen (pancreaticoduodenectomy) of different case: ductal adenocarcinoma (asterisk), causing dilation of both the common bile duct (C) and the Wirsung duct (W)
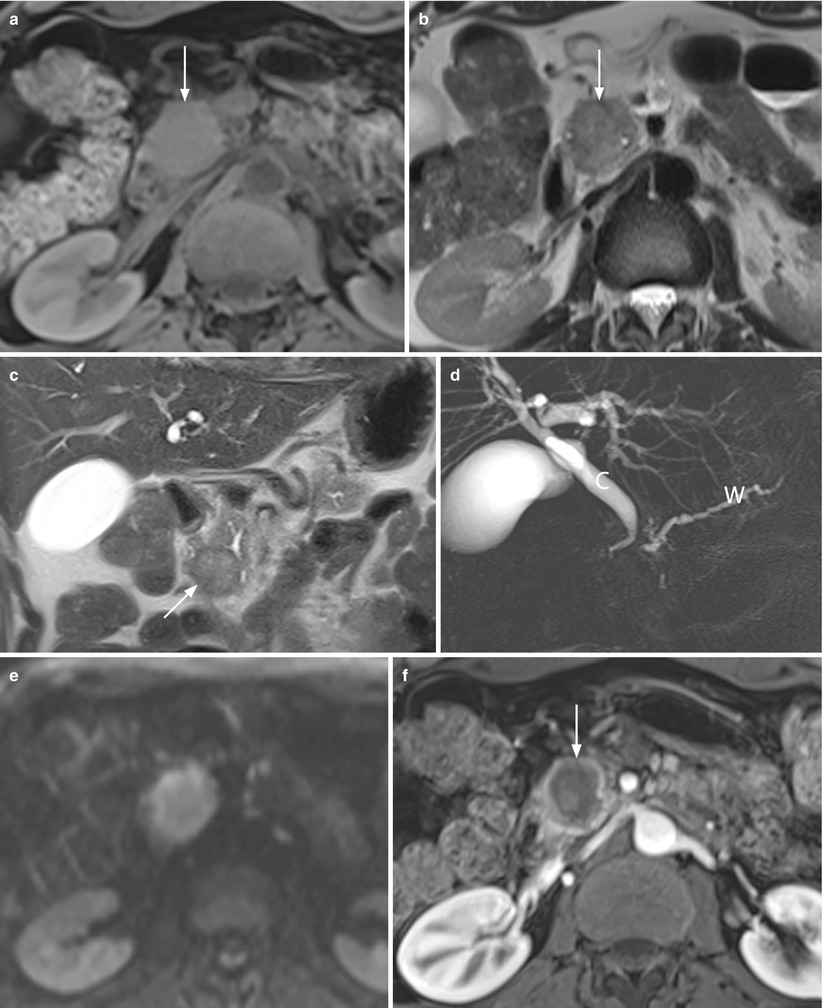
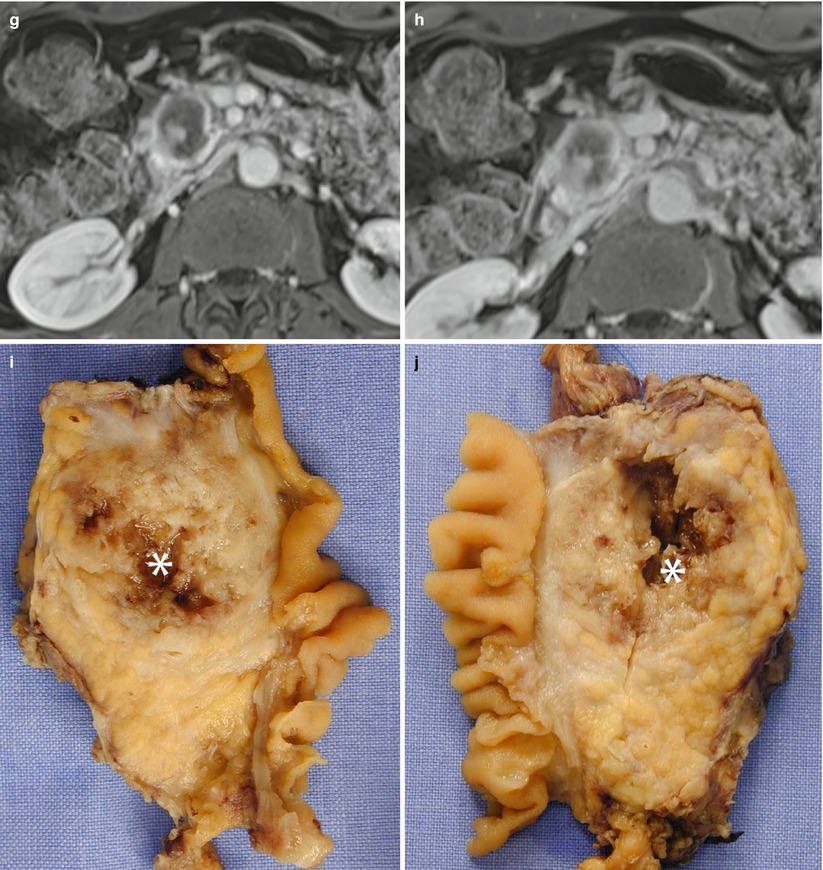
Fig. 20
Double-duct sign in pancreatic head necrotic adenocarcinoma. (a-h) MRI study: round-shaped pancreatic head mass with regular margins, appearing slightly hypointense (arrow in a) on T1-weighted fat-saturated images and slightly hyperintense (arrow in b and c) on T2-weighted axial (b) and coronal (c) images. MRCP (d) shows a dilated common bile duct (C in d) and Wirsung duct (W in d) (double-duct sign). The mass shows peripheral diffusion restriction with mild central T2-shine through effect (necrotic component) (e). At dynamic study (f–h), the lesion appears markedly hypovascular (arrow in f) with avascular portions in the pancreatic (f), venous (g), and late (h) phases. (i, j) Surgical specimen (pancreaticoduodenectomy) of different case: ductal adenocarcinoma with intralesional cystic necrotic portion (asterisk in i and j)
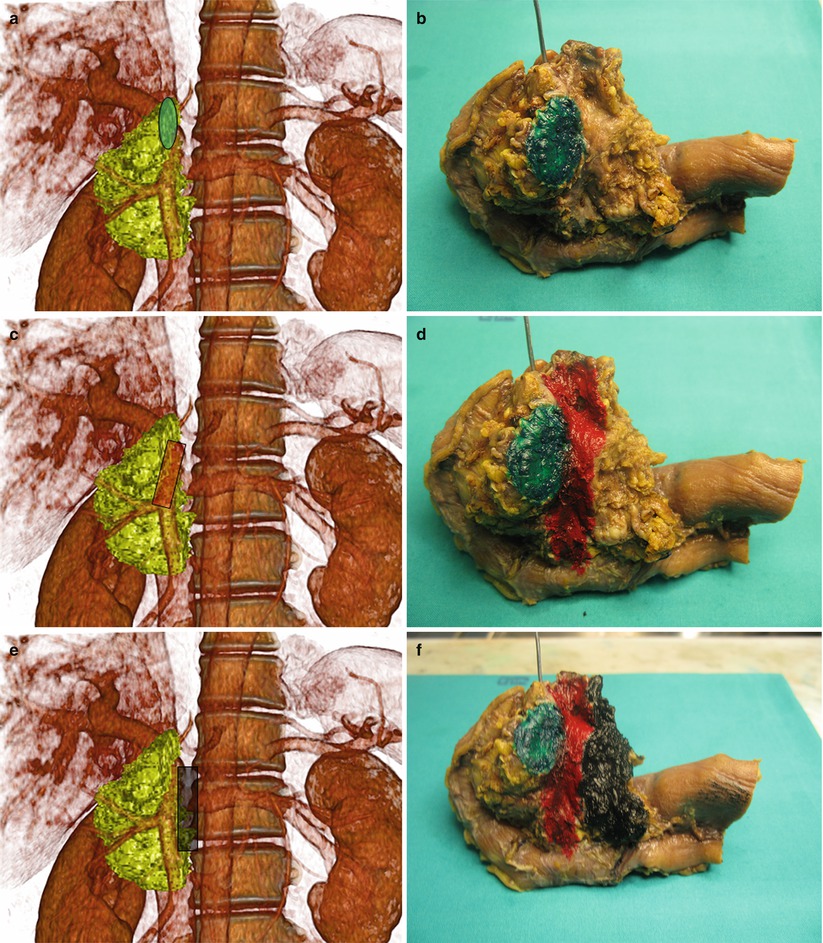
Fig. 21
CT virtual pancreaticoduodenectomy and pathological correlation. (a, b) CT–pathological correlation: volume-rendering (a) of the right pancreas after virtual resection with pancreatic neck margin highlighted (green). Pancreaticoduodenectomy (b), with the pancreatic margin highlighted (green) on the surgical specimen. (c, d) CT–pathological correlation: volume-rendering (c) of the right pancreas after virtual resection with the vascular bed, where the superior mesenteric vein/portal vein and the superior mesenteric artery originally lie, highlighted (red). Pancreaticoduodenectomy (d), with the vascular bed highlighted (red) on the surgical specimen. (e, f) CT–pathological correlation: volume-rendering (e) of the right pancreas after virtual resection with pancreatic posterior retroperitoneal margin highlighted (black). Pancreaticoduodenectomy (f), with the pancreatic posterior retroperitoneal margin highlighted (black) on the surgical specimen. Probe in the common bile duct
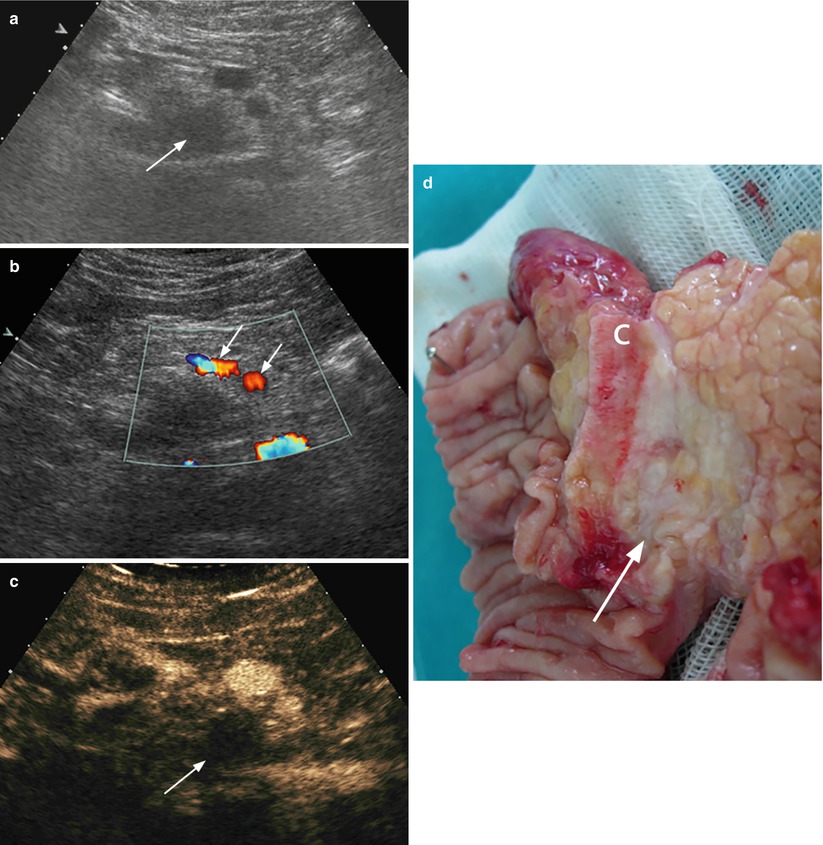
Fig. 22
Resectable ductal adenocarcinoma. (a–c) Ultrasound study: conventional examination (a) shows a hypoechoic round-shaped lesion (arrow) in the pancreatic head with regular aspect of the superior mesenteric vessels (arrows in b). At CEUS (c), the lesion is typically markedly hypovascular (arrow). (d) Surgical specimen (pancreaticoduodenectomy): small adenocarcinoma (arrow), confined in the pancreatic head periampullary region. Common bile duct (C)
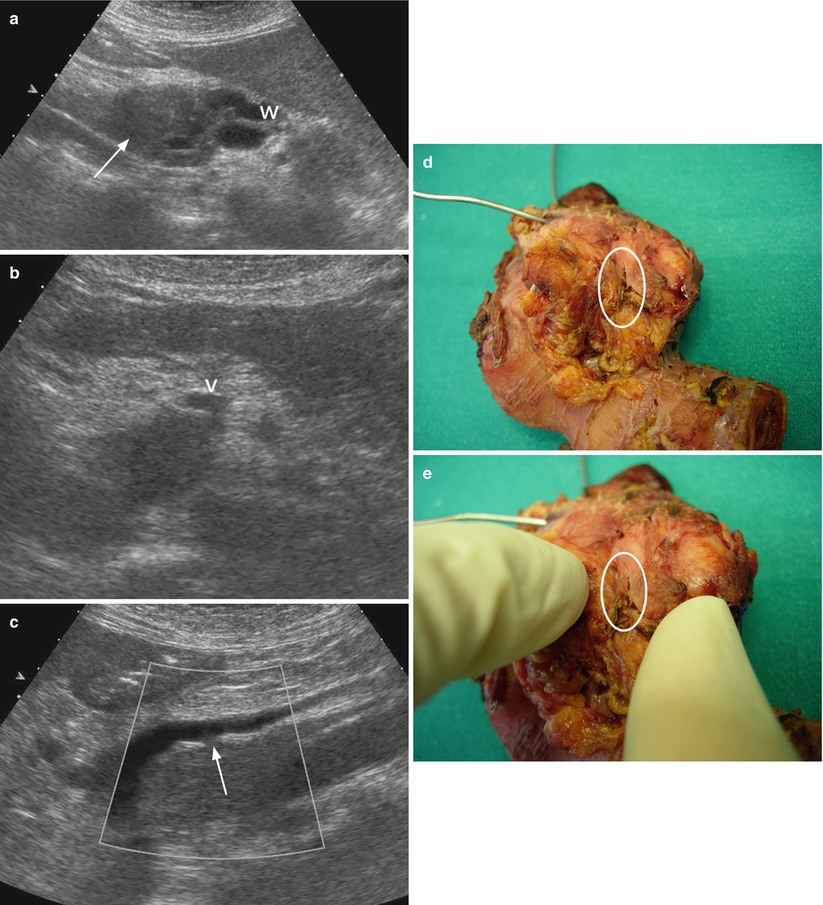
Fig. 23
Ductal adenocarcinoma with small venous infiltration. (a–c) Ultrasound study: conventional examination shows hypoechoic ill-defined mass (arrow in a) in the pancreatic head with main duct (W) upstream dilation. The mass involves the uncinate process infiltrating the posterior wall of the superior mesenteric vein (V in b). At longitudinal scan (c) with Doppler clarification is clearly visible the small infiltration (arrow in c) of the superior mesenteric vein wall. (d, e) Surgical specimen (pancreaticoduodenectomy): on the posterior surface of the surgical specimen, a small irregularity of the vascular bed (circle) is documented and better visible on the detail image (e). Probes in the Wirsung duct and in the common bile duct are visible
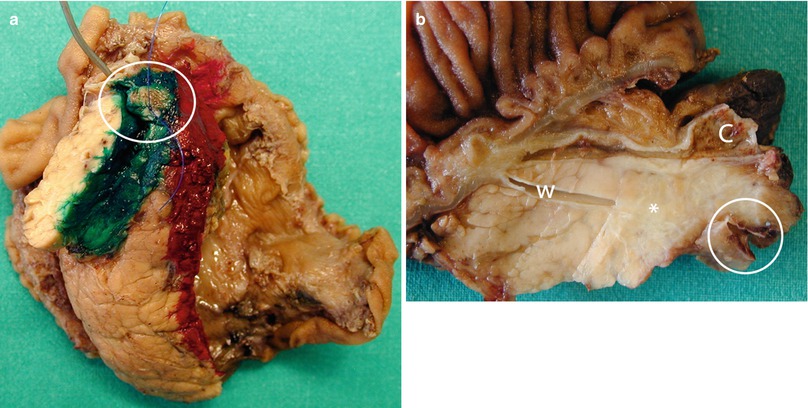
Fig. 24
Ductal adenocarcinoma and vessels infiltration. (a) Surgical specimen (pancreaticoduodenectomy): on the posterior surface of the specimen, partial vascular resection (circle) is visible along the vascular bed (green). (b) Surgical specimen: ductal adenocarcinoma (asterisk), involving the main pancreatic duct (W) and the common bile duct (C) with superior mesenteric vein resection (circle)
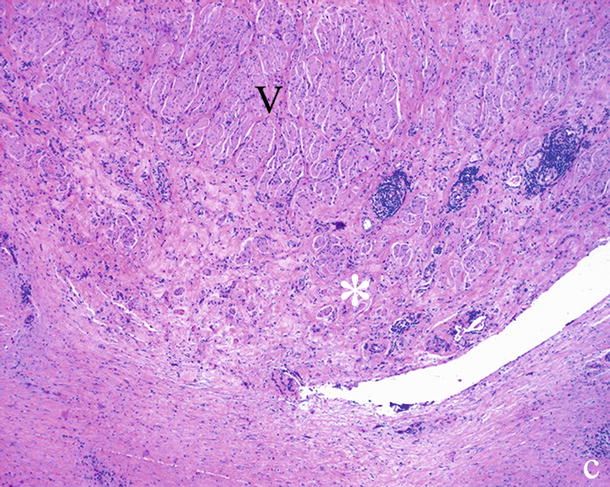
Fig. 25
Ductal adenocarcinoma and inflammatory vessel involvement. Histopathology: fibro-inflammatory (asterisk) adhesion to the resected portion of superior mesenteric vein (V)
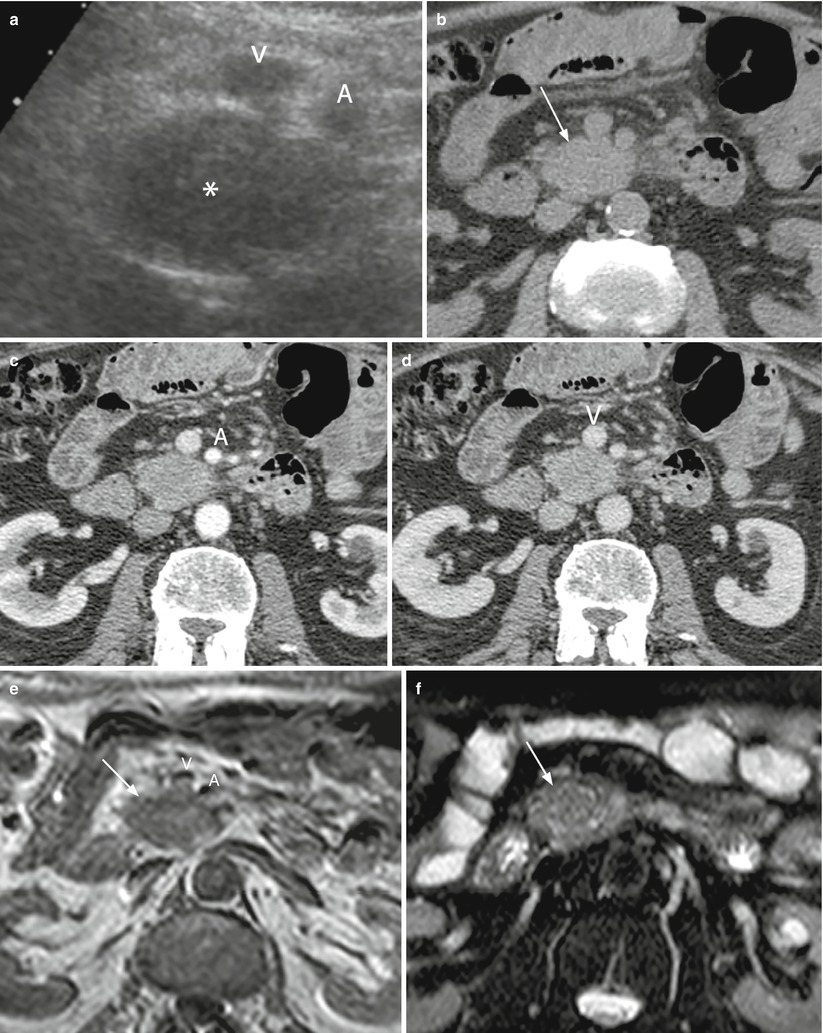
Fig. 26
Borderline resectable ductal adenocarcinoma. (a) Conventional ultrasound: hypoechoic solid mass (asterisk) of the uncinate process of the pancreas with small infiltration of the superior mesenteric vein (V) and in contact with the superior mesenteric artery (A). (b–d) CT study: solid mass of the uncinate process of the pancreas, isodense (arrow in b) in the baseline study, and hypodense in the pancreatic (c) and venous (d) phases in contact with the superior mesenteric artery (A in c) and vein (V in d). (e, f) MRI study: solid mass of the uncinate process of the pancreas, slightly hypointense on T1- (arrow in e) and T2- (arrow in f) weighted images, with small infiltration of the superior mesenteric vein (V in e), and in contact with the superior mesenteric artery (A in e)
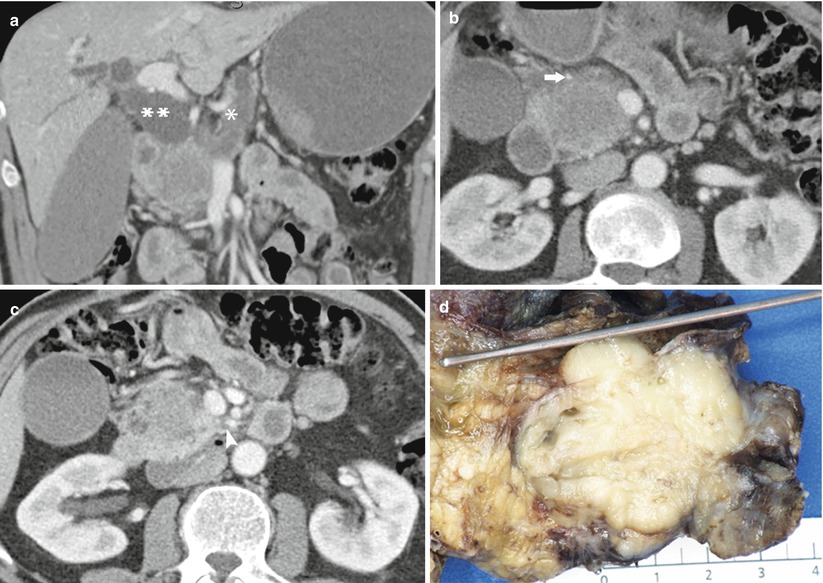
Fig. 27
Borderline resectable pancreatic head ductal adenocarcinoma. (a) The main pancreatic duct and common bile duct (**) are distended. The intrahepatic bile ducts and gallbladder are dilated. (b ) The mass in the head of the pancreas shows low attenuation on the pancreatic phase. The mass contacts the gastroduodenal artery (arrow). The superior mesenteric vein is smoothly compressed. The superior mesenteric artery appears free of tumor contact. (c) Image slightly more inferior reveals vague increased density in the fat in the vicinity of the superior mesenteric artery (arrowhead). (d) Pathologic specimen from Whipple’s resections shows a whitish tumor mass within the head of the pancreas. The probe is in the dilated common bile duct. A dilated main pancreatic duct is visualized. The tumor was 4.5 cm, moderately differentiated with microscopic extension into the peripancreatic soft tissue. Lymphovascular and perineural invasions were present. This finding could be suspected by the unclear margins along the inferior portion of the superior mesenteric artery (b, c)
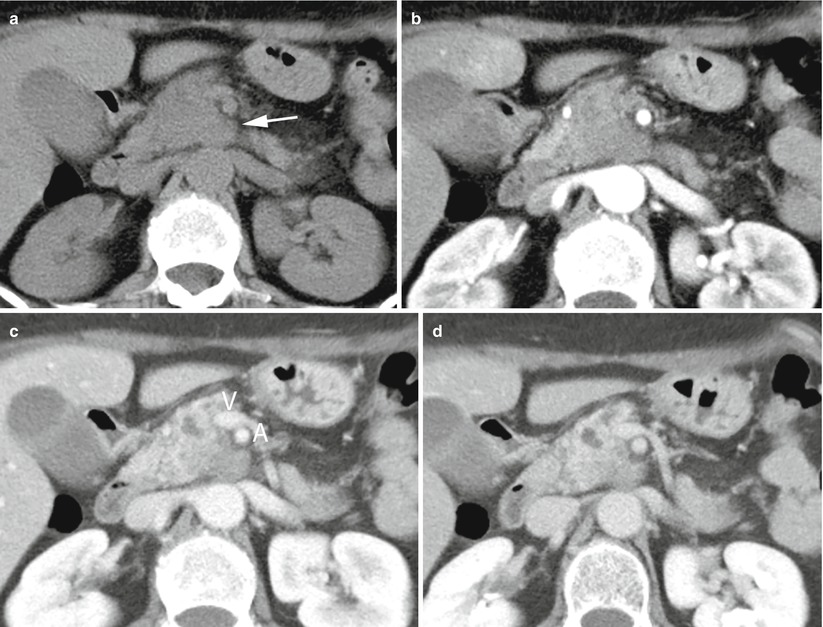
Fig. 28
Borderline resectable ductal adenocarcinoma. (a–d) CT study: solid mass of the uncinate process of the pancreas, isodense (arrow in a) in the baseline study and hypodense in the pancreatic (b), venous (c), and late (d) phases in contact with the superior mesenteric vein (V in c); hyperdensity of the fat next to the superior mesenteric artery (A in c) can be seen
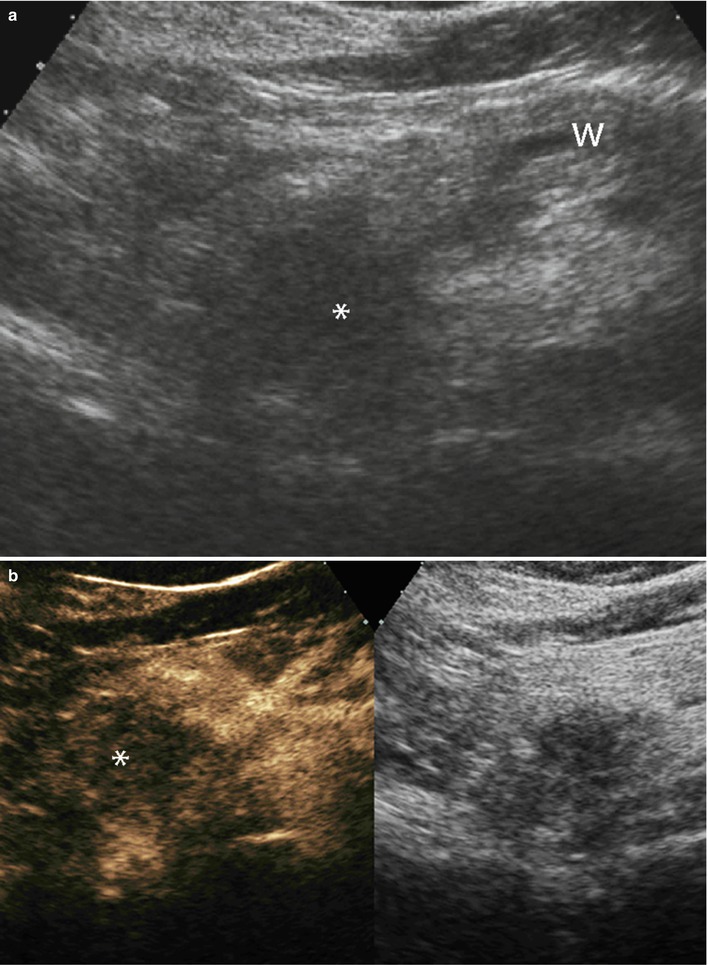
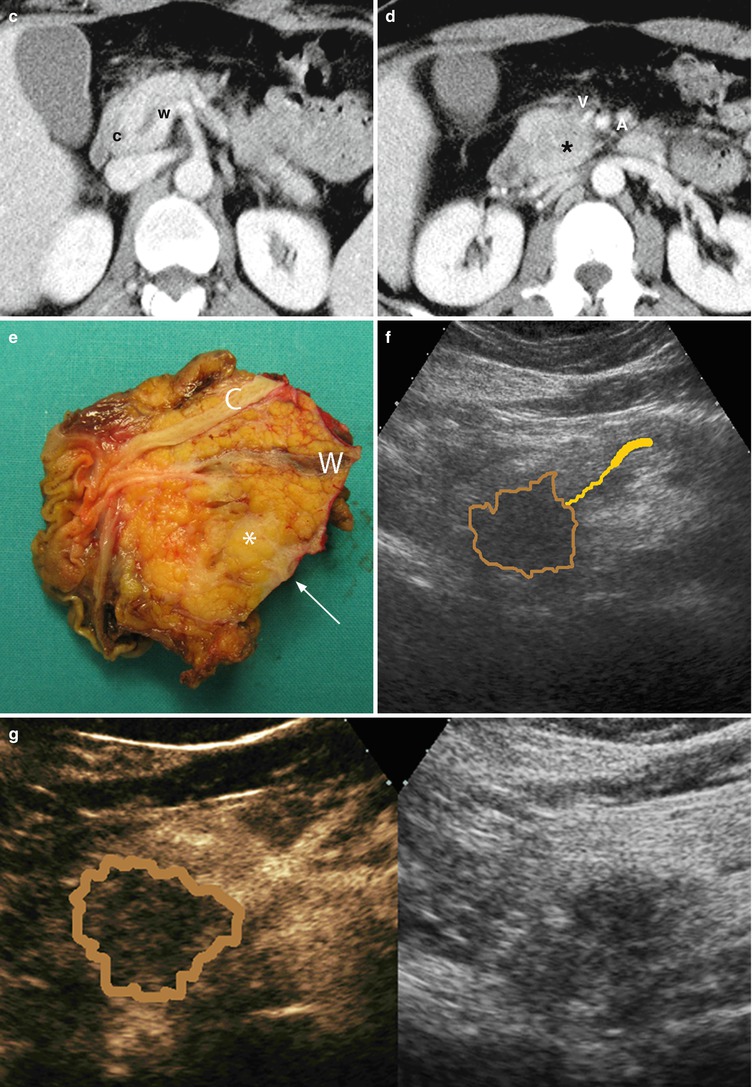
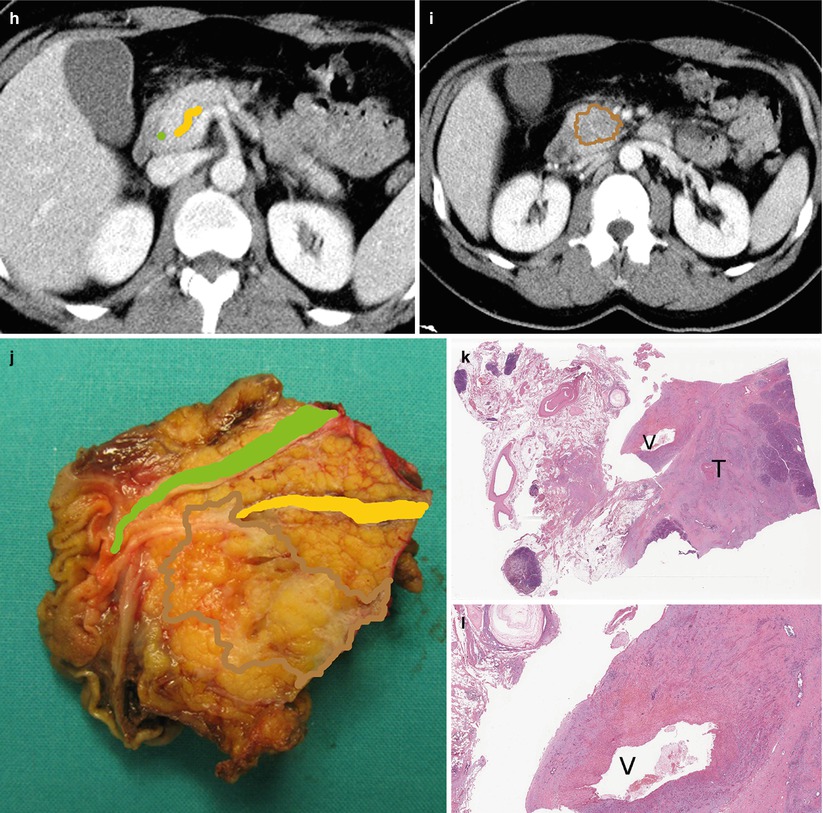
Fig. 29
Ductal adenocarcinoma with infiltration of superior mesenteric vein. (a, b) Ultrasound study: hypoechoic solid mass (asterisk in a) in the uncinate process of the pancreas with main pancreatic duct (W in a) dilation. The mass results typically markedly hypovascular (asterisk in b) at CEUS. (c, d) CT study: dilation of the Wirsung duct (W in c) caused by a hypodense mass (asterisk in d) in the uncinate process of the pancreas. Common bile duct (C in c) is normal. The mass infiltrates the superior mesenteric vein (V in d) and is very close to the superior mesenteric artery (A in d). (e) Surgical specimen (pancreaticoduodenectomy): ductal adenocarcinoma (asterisk) involving the Wirsung duct (W). The common bile duct (C) is normal. The pancreatic surface of the vascular bed is involved (arrow). (f–j) Superimposed drawings better clarify the lesion (brown circle), the Wirsung duct (yellow line), and the common bile duct (green line) in the imaging and surgical specimen. (k–l) Histopathology: the tumor (T in k) involves the superior mesenteric vein (V in k and l) resected
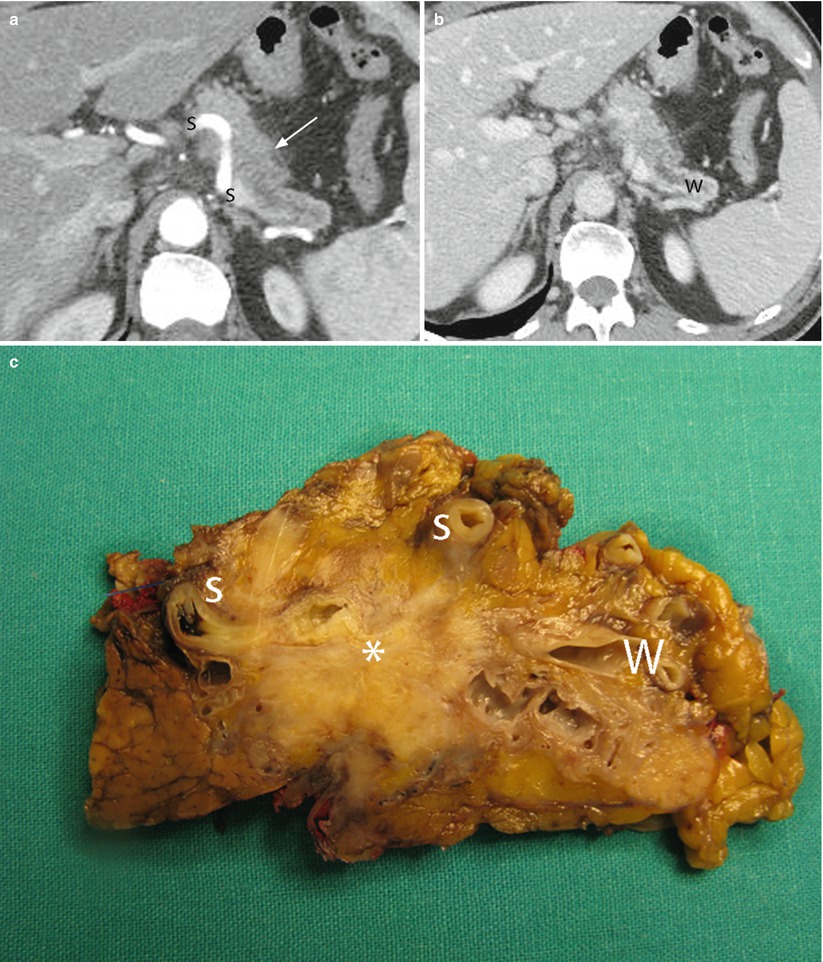
Fig. 30
Ductal adenocarcinoma with infiltration of the splenic artery. (a, b) CT study: solid mass hypodense (arrow in a) in the pancreatic (a) and venous (b) dynamic phases in the body of the pancreas with main pancreatic duct (W in b) dilation. The mass infiltrates the splenic artery (S in a). (c) Surgical specimen (distal pancreatectomy): ductal adenocarcinoma (asterisk) with dilation of the Wirsung duct (W) infiltrating the splenic artery (S)
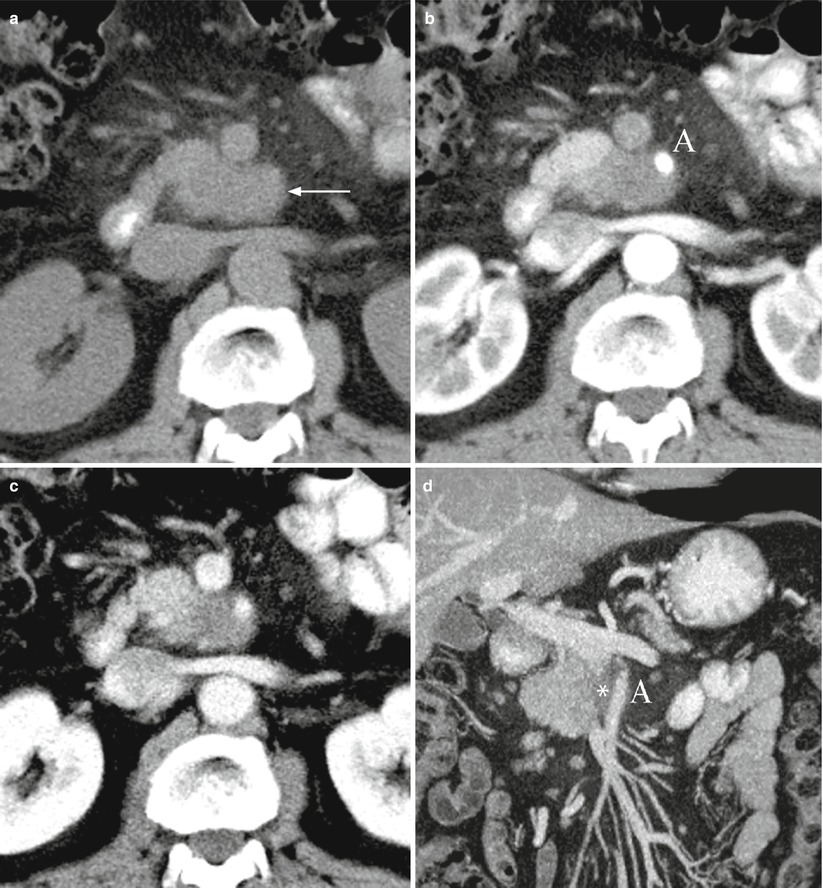
Fig. 31
Ductal adenocarcinoma with infiltration of the superior mesenteric artery. (a–d) CT study: solid mass in the uncinate process of the pancreas, isodense on baseline (arrow in a) and hypodense in the pancreatic (b) and venous (c) dynamic phases. The mass infiltrates the superior mesenteric artery (A in b and d) with long extension along this vessel (asterisk in d), better visible on multiplanar-reconstructed coronal image (d)
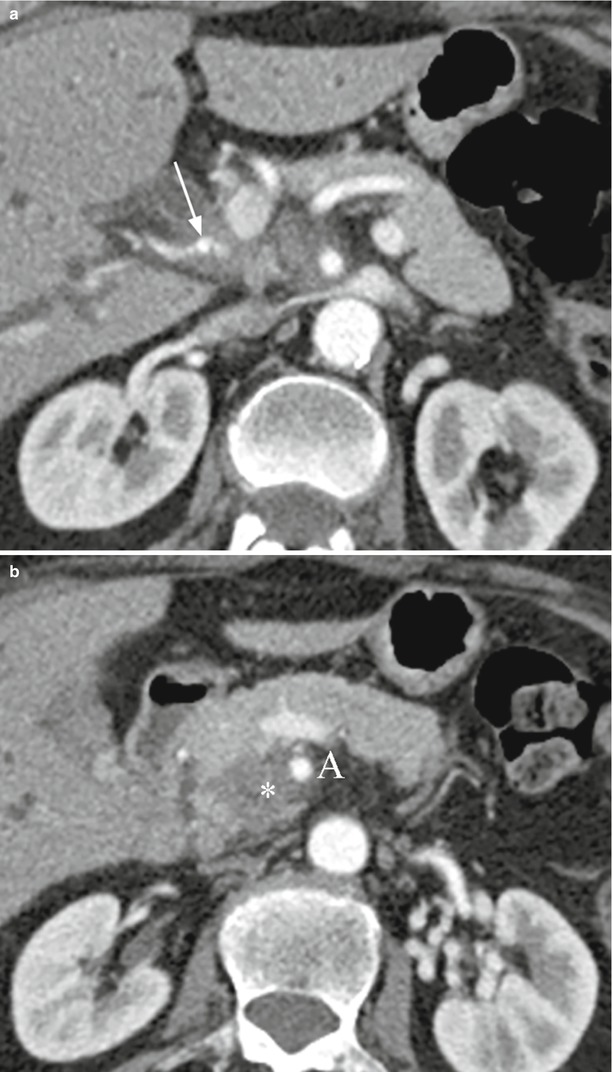
Fig. 32
Ductal adenocarcinoma with arterial infiltration. (a, b) CT study: hypodense neoplastic tissue, infiltrating the hepatic artery (arrow in a) originating from the superior mesenteric artery, is growing from a hypodense mass (asterisk in b) of the uncinate process of the pancreas. The mass infiltrates also the superior mesenteric artery (A in b)
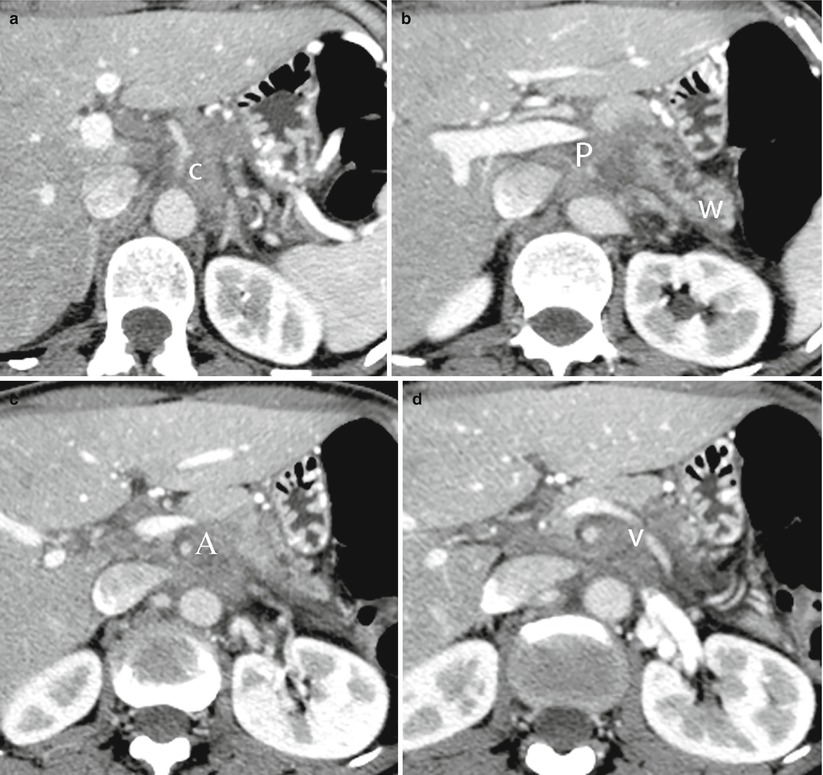
Fig. 33
Ductal adenocarcinoma with retroperitoneal spread and vessels infiltration. (a–d) CT study: hypodense neoplastic tissue involving diffusely the retroperitoneum, infiltrating the celiac trunk (C in a), the portal vein (P in b), superior mesenteric artery (A in c), and the splenic vein (V in d). The main pancreatic duct (W in b) is dilated
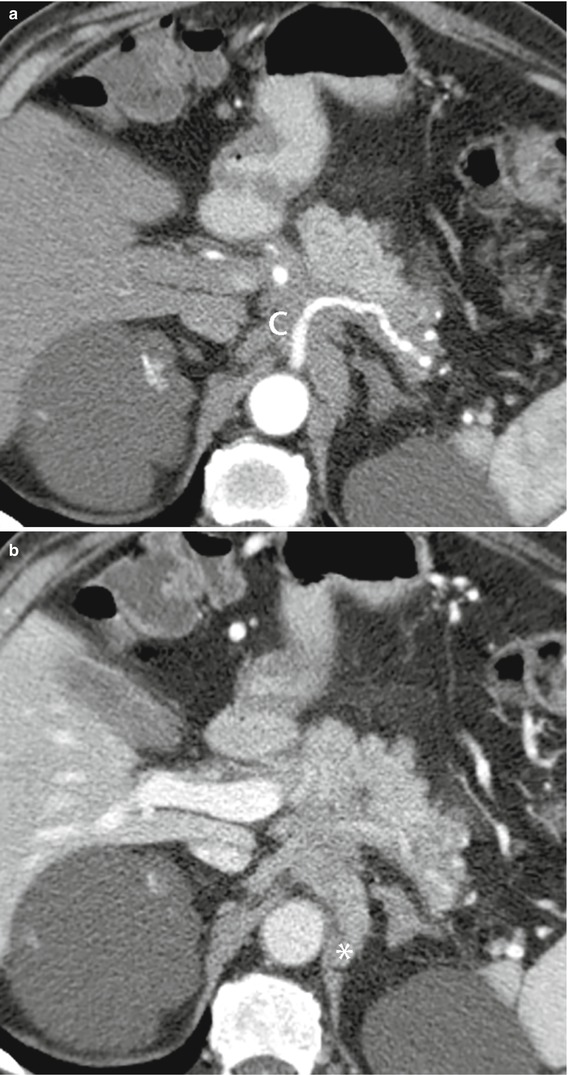
Fig. 34
Ductal adenocarcinoma with retroperitoneal spread and vessels infiltration. (a, b) CT study: neoplastic tissue hypodense in the pancreatic (a) and venous (b) dynamic phases involving diffusely the retroperitoneum infiltrating the celiac trunk (C in a) and the diaphragmatic pillar (asterisk in b)
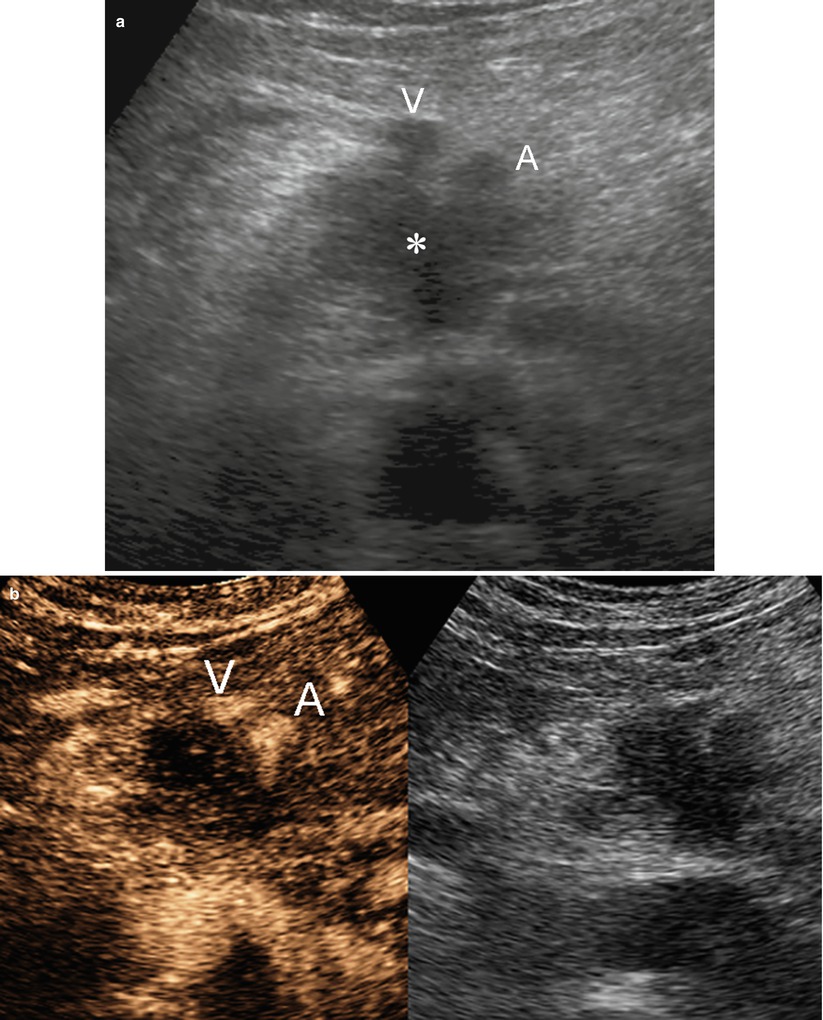
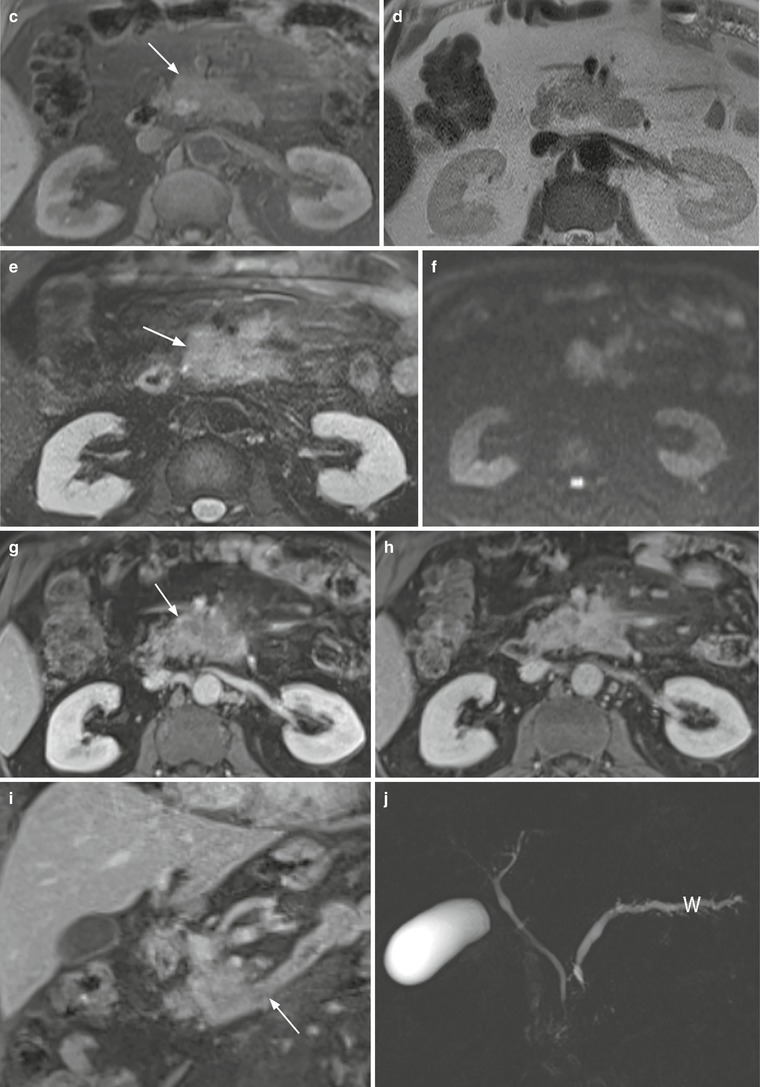
Fig. 35
Ductal adenocarcinoma with superior mesenteric vessels infiltration. (a, b) Ultrasound study: hypoechoic mass (asterisk in a) of the uncinate process of the pancreas, resulting markedly hypovascular at CEUS. The mass infiltrates the superior mesenteric vein (V in a and b) and artery (A in a and b). (c–j) MRI study: uncinate process pancreatic mass, hypointense on T1-weighted fat-saturated image (arrow in c) and slightly hyperintense on T2-weighted images, both non–fat saturated (d) and fat-saturated (arrow in e). The mass shows diffusion restriction on DWI (f) with high b value. In the pancreatic dynamic phase, the mass is hypovascular (arrow in g) with progressive contrast medium retention in the following phases (h and i). In the coronal acquisition, the involvement of the duodenum and the Treitz ligament is visible (arrow in i). On MRCP (j), the Wirsung duct (W) is dilated
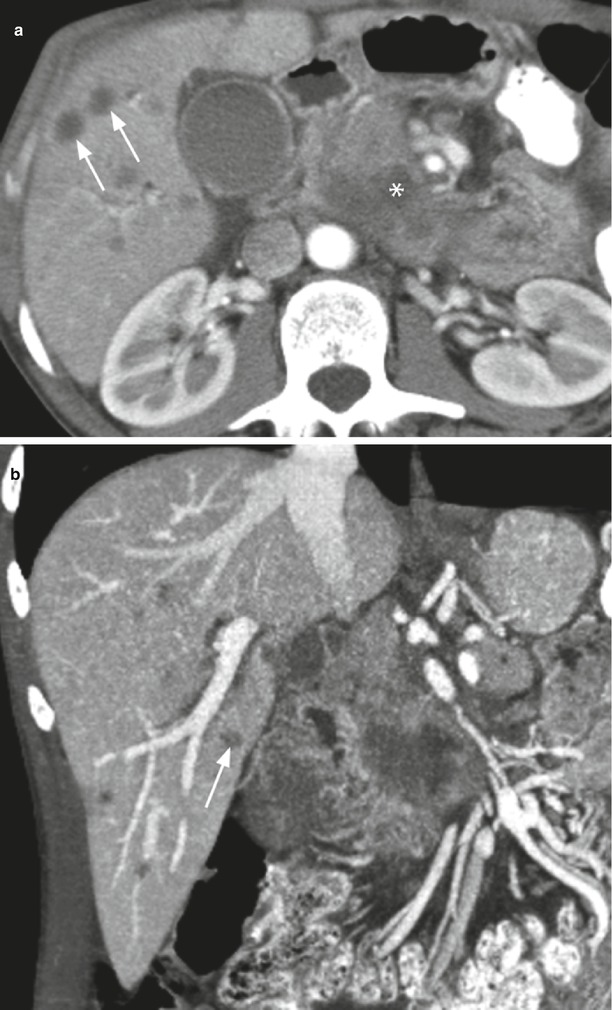
Fig. 36
Ductal adenocarcinoma with metastatic spread. (a, b) CT study: hypodense uncinate process mass (asterisk in a). Liver metastases (arrows in a and b) are visible
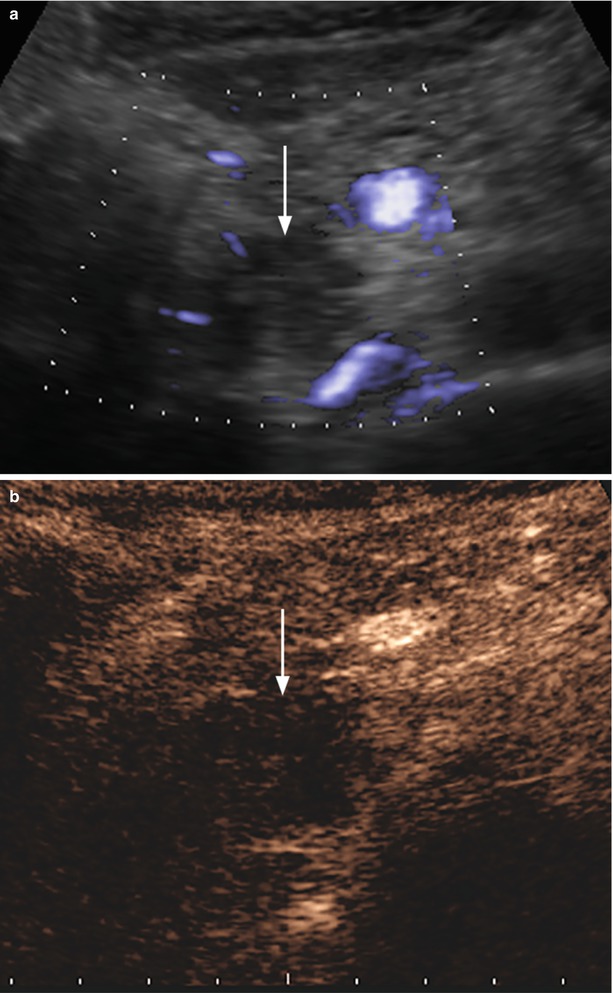
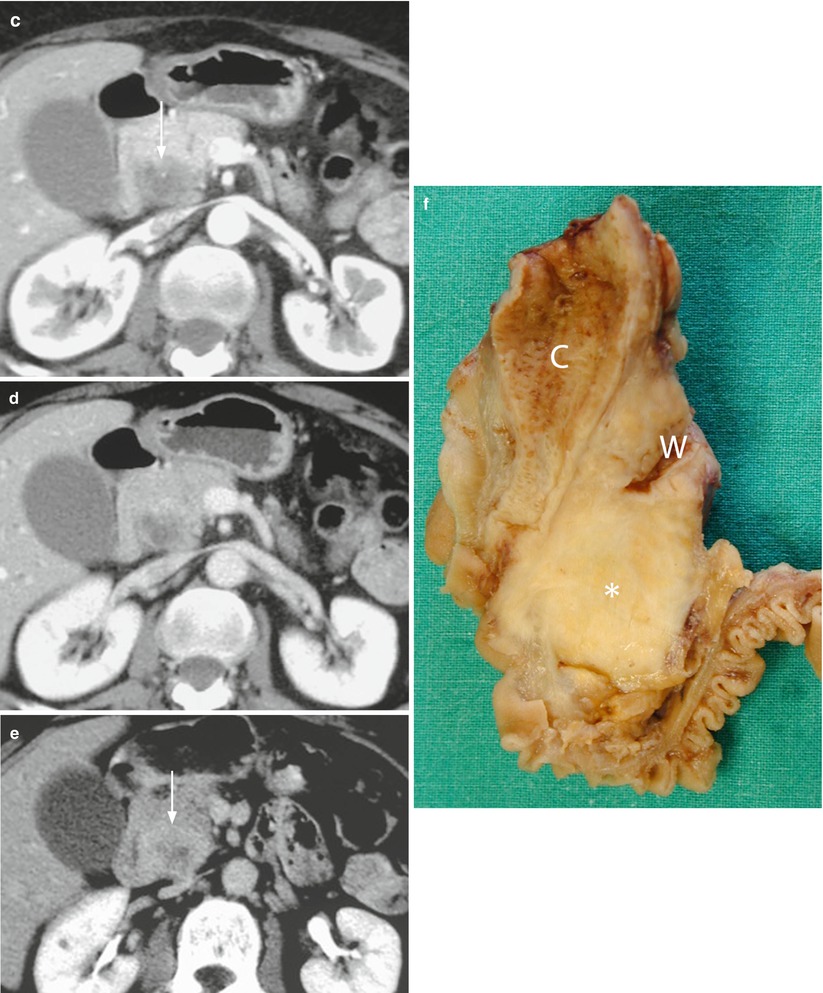
Fig. 37
Ductal adenocarcinoma. (a, b) Ultrasound study: hypoechoic mass (arrow in a) of the pancreatic head with no intralesional color Doppler signal, resulting markedly hypovascular (arrow in b) at CEUS. (c–e) CT study: the pancreatic head mass is hypodense (arrow in c) in the pancreatic (c) and venous (d) dynamic phases, with progressive contrast medium retention, due to accumulation in the fibrotic tissue, clearly visible in the late phase (e), appearing inhomogeneously hyperdense (arrow in e). (f) Surgical specimen (pancreaticoduodenectomy) of different case: highly fibrotic ductal adenocarcinoma (asterisk) with dilation of the Wirsung duct (W) and the common bile duct (C)
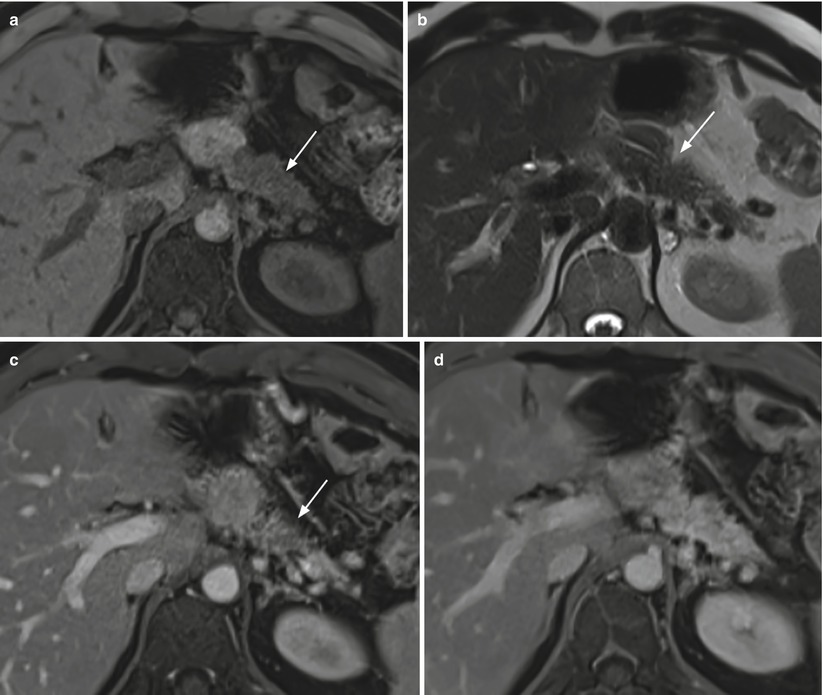
Fig. 38




Ductal adenocarcinoma. (a–d) MRI study: pancreatic mass with elongated morphology, diffusely involving the body–tail region, appearing hypointense (arrow in a) on T1-weighted fat-saturated images and isointense on T2-weighted (b) non-fat saturated images. Ventral border retraction (arrow in b) is visible at the pancreatic body. At dynamic MRI, the mass is hypovascular (arrow in c) in the pancreatic (c) phase, with progressive contrast medium retention starting from the venous (d) phase
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree