Key points
- •
Tinnitus is associated with complex alterations to primary auditory, sensory, and limbic networks.
- •
Functional MR imaging and diffusion tensor imaging are capable of revealing such changes. This may aid in the identification of neural targets amenable to modification by novel treatment strategies.
- •
Newer MR imaging techniques that depict changes to the labyrinth in Meniere disease have emerged and may be of value in diagnosis in equivocal cases.
- •
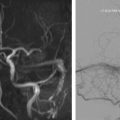
Advances in computed tomography and MR imaging techniques have improved the ability to noninvasively diagnose dural arteriovenous fistulae.
Introduction
Previous articles have mostly dealt with the use of imaging techniques in the evaluation of structural causes of tinnitus. However, in most tinnitus sufferers, whose symptoms are subjective, routine imaging studies are unrevealing. It is now well known that complex alterations in auditory and nonauditory neural circuits underlie the genesis and perception of tinnitus and its associated symptoms in such patients. Advanced structural and functional neuroimaging techniques have shed new light on these alterations. Although techniques such as PET and magnetoencephalography have also been used in an attempt to understand these changes, this article reviews functional MR imaging (fMR imaging) and diffusion tensor imaging (DTI) techniques. A brief discussion of the use of advanced MR imaging techniques in the diagnosis of Meniere disease (MD) and dural arteriovenous fistulae (DAVF) is also presented.
Introduction
Previous articles have mostly dealt with the use of imaging techniques in the evaluation of structural causes of tinnitus. However, in most tinnitus sufferers, whose symptoms are subjective, routine imaging studies are unrevealing. It is now well known that complex alterations in auditory and nonauditory neural circuits underlie the genesis and perception of tinnitus and its associated symptoms in such patients. Advanced structural and functional neuroimaging techniques have shed new light on these alterations. Although techniques such as PET and magnetoencephalography have also been used in an attempt to understand these changes, this article reviews functional MR imaging (fMR imaging) and diffusion tensor imaging (DTI) techniques. A brief discussion of the use of advanced MR imaging techniques in the diagnosis of Meniere disease (MD) and dural arteriovenous fistulae (DAVF) is also presented.
Neural correlates of tinnitus
Although tinnitus may originate from damage to peripheral auditory structures, it is well established that the perception of the phantom sound is largely the result of central neural processes. Evidence for a central origin of tinnitus stems from the finding that tinnitus is an invariable consequence of sectioning the auditory nerve, as may occur with acoustic neuroma surgery, and in such patients, tinnitus does not resolve and may actually worsen after the procedure. Damage to hair cells of the inner ear related to age and noise exposure is the inciting process in most patients. Tinnitus is believed to arise from an abnormal neuroplastic response to this process, which manifests as hyperactivity of neurons at multiple levels in the auditory pathway. This hyperactivity may be the result either of central downregulation of inhibition, or possibly from upregulation of excitatory inputs. Reorganization of the auditory cortical tonotopic map also is believed to occur. This results from cortical neurons deprived of auditory thalamocortical input responding to input from their unaffected neighbors. The mechanisms responsible for how the phantom sound is consciously perceived remain elusive. Llinás and colleagues propose that aberrant thalamocortical rhythms (thalamocortical dysrhythmia) that may emerge as a plastic maladaptation to peripheral auditory injury may generate the conscious percept of tinnitus. Changes in the brain with tinnitus are not confined to the auditory pathway. Roberts and colleagues discuss the role of the somatosensory system in the modulation of the tinnitus percept, given that patients with tinnitus can alter their perception of the phantom sound by somatic maneuvers such as jaw clenching or tensing their neck muscles. It is not surprising that changes in the limbic system are a feature of chronic tinnitus. The hippocampus, nucleus accumbens, and amygdala, among others, seem to play a key role in the symptoms of anxiety, sleeplessness, and impairment of mood and memory that chronic tinnitus sufferers experience. For detailed discussion on the neural underpinnings of tinnitus, (See Ryan D, Bauer CA: Neuroscience of tinnitus , in this issue.)
Functional MR imaging in tinnitus
Since the discovery of the functional significance of spontaneous low-frequency blood oxygen level–dependent (BOLD) signal fluctuations (less than 0.1 Hz) in the brain by Biswal and colleagues, the use of resting-state fMR imaging (rsfMR imaging) in neurologic disorders has greatly expanded. In this study, using a seed-based approach, a high degree of correlation in the temporal course of fluctuations between the left somatosensory cortex at rest and homologous areas in the right hemisphere was observed, comprising a functionally connected sensorimotor network. The physiologic basis for these temporal fluctuations is not entirely clear but seems to be tightly coupled with neural activity. Several other resting-state networks (RSNs) have been subsequently discovered. Of these, the default mode network (DMN) discovered by Raichle and colleagues on PET imaging, and then by Greicius and colleagues on rsfMR imaging, is perhaps the best known. In addition, RSNs for language, memory, vision, hearing, and attention, among others, have been identified and examined in depth.
Several methods may be used to extract networks from resting-state data. After undergoing a series of preprocessing steps, including correction for section-dependent time shifts and intensity differences, head motion, nuisance regression (related to cardiac and respiratory motion), spatial smoothing, application of low-pass filters, and registration to standardized atlas space, data may be analyzed using a seed-based approach, independent component analysis (ICA), or graph methods among other techniques. Each of these has its strengths and weaknesses. The seed-based approach, in which a region of interest (ROI) is placed over the area in question and its BOLD time series correlated with all other voxels in the brain, is perhaps the most frequently used method. However, this approach requires a priori selection of ROIs. ICA is also a widely used method that is perhaps statistically more robust, requiring fewer a priori assumptions. These approaches are comparable. Rosazza and colleagues in a resting-state study of 40 participants found significant (though not complete) correspondence between these methods. Graph theory methods allow representation of ROIs as a network of nodes and edges from which several connectional characteristics may be inferred. Graph methods have revealed that the brain’s intrinsic activity is organized as a small-world, highly efficient network, with significant modularity and highly connected hub regions.
fMR imaging studies in tinnitus reveal 2 broad themes: correlation differences between auditory and nonauditory brain networks, and an increased correlation between the limbic system and other brain areas. These, discussed in detail in an excellent review of the topic by Husain and Schmidt, reflect to a large extent what is now understood about the neural correlates of tinnitus. Aberrant neural activity in auditory and nonauditory regions has been described in numerous studies. In a task-based fMR imaging study, Golm and colleagues describe increased activity in a fronto-parietal-cingulate network, which seems to be more active in highly distressed tinnitus patients. In a group of 13 patients with chronic tinnitus, Maudoux and colleagues describe increased connectivity in extra-auditory regions such as the brainstem, cerebellum, nucleus accumbens, parahippocampal gyrus, and sensorimotor cortex, indicating a modification of cortical and subcortical functional connectivity to include areas that participate in attention, memory, and emotional processes.
Support for alterations in memory networks may also be found in single-photon emission computed tomography (SPECT) studies. For example, Laureano and colleagues describe significantly increased perfusion in the parahippocampal gyrus using SPECT, in a series of 20 subjects with chronic tinnitus and normal hearing. Interactions between the auditory and limbic systems using fMR imaging have been revealed in studies by Kim and Horwitz, and discussed in a review by Rauschecker and colleagues. The role of visual and attentional networks has been explored by Burton and colleagues. In a study of 17 subjects with bothersome tinnitus, negative correlations reciprocally characterized functional connectivity between auditory and occipital-visual cortex. Connectivity for primary visual cortex in tinnitus included extensive negative correlations with regions that participate in the executive control network. The investigators interpret this dissociation in activity between the auditory cortex and visual and attention-control networks in 2 ways: either the reciprocal negative correlations in connectivity between these networks are a maladaptation or they reflect an adaptive response to suppress the salience of the phantom sound and minimize conflict with attentional tasks. In an rsfMR imaging study by Schmidt and colleagues, in subjects with tinnitus and mild-to-moderate high-frequency hearing loss, the DMN was the only RSN to display visual differences between subjects with tinnitus and controls. In the DMN, strongly decreased correlation was identified between seed regions and the precuneus. Using a seed-based approach, in the same study, increased connectivity was noted between limbic areas (the left parahippocampal gyrus) and the auditory RSN and the attention network (right parahippocampal gyrus).
The investigators suggest that treatment strategies aimed at relieving tinnitus-related distress target increased limbic-auditory or attention network connectivity, as well as the decreased coherence of the DMN. Disruption of the DMN may be attributable to changes in the middle temporal and angular gyri, as evidenced by increased amplitude of low-frequency fluctuation (ALFF), a measure that may reflect the intensity of regional spontaneous brain activity found in these areas by Chen and colleagues. They also found increased ALFF in the superior frontal gyrus, a finding that correlated with the duration of tinnitus. This may lend support to the hypothesis that the frontal cortex may be a vital structure subserving the tinnitus mechanism, as proposed by Jastreboff. Chen and colleagues also found decreased ALFF values in the visual cortex and thalami. The decreased visual cortical ALFF may be a consequence of suppression of the visual network by the phantom sounds, whereas the thalamic alterations may be a manifestation of the thalamocortical dysrhythmia hypothesis of Llinás and colleagues. Fig. 1 provides a summary of recent fMR imaging studies in tinnitus.
It must be stressed that the findings of fMR imaging studies must be interpreted with a measure of caution. Variations in the analytical methods used (eg, seed-based methods vs ICA) in the anatomic locations of ROIs studied and in the numbers of subjects evaluated may all affect results. Husain and colleagues recommend that researchers focused on the use of rsfMR imaging in tinnitus voluntarily adopt a more standardized approach in the design of their fMR imaging studies. The effect of mechanical noise intrinsic to MR imaging scanners, despite the use of noise reduction strategies, also cannot be disregarded. However, the most important variable may be the heterogeneity in the clinical characteristics of subjects studied. These may lie in demographic features (eg, age, gender) or in the clinical characteristics of the tinnitus itself (eg, severity, laterality, associated hearing loss). The importance of recognizing this variability was also recognized by Wineland and colleagues. In a study of 18 subjects with nonbothersome tinnitus, they found no alterations in functional connectivity of the auditory cortex or of other relevant cortical areas. This was in contrast to an earlier study by Burton and colleagues in which changes in connectivity of sensory and cognitive networks were observed in a cohort of subjects with bothersome tinnitus. The investigators stress that imaging studies that recognize the heterogeneity of the cognitive and emotional symptoms that accompany tinnitus may be more informative than those that do not.
Advanced structural imaging
In one of the earliest studies exploring the role of DTI in tinnitus, Lee and colleagues described a small, yet statistically significant decrease in fractional anisotropy (FA) values in the left frontal and right parietal arcuate fasciculi in a group of 28 patients. They postulated that the percept of tinnitus may be related to alterations in specific white matter pathways. Of these, notably, the arcuate fasciculus links areas in the auditory, frontal and limbic cortex, all of which are deemed important in the genesis of the tinnitus percept. The reduction in FA in the left arcuate fasciculus may imply a “lack of harmony in information processing,” whereas changes in the right arcuate fasciculus may implicate the role of attention-related processes. A key limitation of this study was that patients with hearing loss were not evaluated separately from those without.
In a more homogeneous group of subjects, Aldhafeeri and colleagues found significant correlations between cortical thickness reductions in the bilateral temporal and frontal lobes of subjects with tinnitus. This reduction was present to a greater degree in subjects with associated hearing loss. These differences were most evident in the prefrontal cortex, temporal lobes, and limbic system. The investigators postulate that the decreased prefrontal cortical thickness, in conjunction with the decreased FA that was found in the inferior fronto-occipital fasciculus, may be central to the deficits in cognition, attention, emotional, and memory deficits experienced by tinnitus sufferers. The investigators also noted decreased FA in the inferior longitudinal fasciculus (which may mediate visual-limbic interactions) and in the corpus callosal splenium, perhaps manifesting as an imbalance between interhemispheric excitation and inhibition. They also describe a reduction in cortical thickness in the auditory cortex, supporting studies by Schneider and colleagues, who used voxel-based morphometry to demonstrate decreased gray matter thickness in Heschl gyrus.
In a DTI study, Crippa and colleagues found increased connectivity between the amygdala and the auditory cortex, implying increased auditory-limbic interaction in tinnitus sufferers. In this small study of 10 subjects, associated hearing deficits were not accounted for. In a 2011 study, Husain and colleagues found that hearing loss, rather than tinnitus itself, may have a more significant impact on gray and white matter changes as detected by voxel-based morphometry and DTI. Studies that effectively combine structural and functional neuroimaging methods to evaluate the neural basis of tinnitus are scarce. In their review, Husain and colleagues stress the need for such work, ideally conducted on a clinically homogeneous population.
Meniere disease
MD is characterized by intermittent episodes of vertigo (with horizontal rotatory nystagmus), fluctuating sensorineural hearing loss, tinnitus, and aural pressure. Intense tinnitus is common in MD and is more often seen in the late stage of the disease. The causes of MD and the pathologic changes in the inner ear during acute exacerbations of MD are incompletely understood. Dilatation of the endolymphatic spaces (hydrops) of the inner ear arising from obstruction to endolymph outflow in the endolymphatic sac and duct is a basic pathologic feature of MD. The histologic processes that affect the sac or duct include perisaccular fibrosis, loss of saccular epithelial integrity, and atrophy or hypoplasia of the vestibular duct. There also seems to be an association between a more anteromedially placed sigmoid sinus and development of MD, perhaps as a result of vascular compression of the endolymphatic sac. A diagnosis of MD may be challenging, given the intermittent nature of its symptomatology. MD may be typical when the full spectrum of cochlear (hearing loss with tinnitus and a sensation of aural pressure) and vestibular (vertigo with aural pressure) symptoms are present and atypical when only 1 or the other is experienced. MD is usually diagnosed by clinical means ( Box 1 ) with a certain diagnosis being possible only when histopathological evidence of endolymphatic hydrops (ELH) is available.
- 1.
Recurrent spontaneous and episodic vertigo. A definitive spell of vertigo lasting at least 20 minutes, often prostrating, accompanied by disequilibrium that can last several days; usually nausea or vomiting, or both; no loss of consciousness. Horizontal rotatory nystagmus is always present.
- 2.
Hearing loss (not necessarily fluctuating)
- 3.
Either aural fullness or tinnitus, or both
Certain MD
Definite disease with histopathological confirmation
Definite MD
Two or more definitive episodes of vertigo with hearing loss, plus tinnitus, aural fullness, or both
Probable MD
Only 1 definitive episode of vertigo and the other symptoms and signs
Possible MD
Definitive vertigo with no associated hearing loss or hearing loss with nondefinitive disequilibrium.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree