Key points
- •
Tinnitus is a common symptom that is heterogeneous in presentation, pathophysiology, and imaging manifestations.
- •
Causes are myriad and a multidisciplinary approach is often required.
- •
Although pulsatile tinnitus is less common, its causes are more readily identified clinically and radiographically.
Background (epidemiology, pathophysiology, and anatomy: clinical perspective)
Tinnitus can be broadly defined as an auditory perception of internal origin. It represents a variety of aural sensations, including high or low frequencies that can be constant or intermittent in character. Tinnitus is not a diagnosis but a symptom with a potpourri of possible causes and correspondingly divergent pathophysiologic, anatomic, diagnostic, and therapeutic considerations. Epidemiologic studies have produced disparate chronic tinnitus prevalence estimates mostly in the range of 8% to 20% in the Western world, the variability likely owing to the heterogeneity of tinnitus and inconsistency with respect to methodology. Although it can present at any point in life, its incidence increases with age as well as in the presence of a variety of comorbidities and other symptoms, including hearing loss. It can be a source of persistent angst in a subset of patients and in 20% of tinnitus sufferers the severity is such that their quality of life is significantly impaired. This article aims to provide a summary of the imaging findings of structural causes of tinnitus, several of which are discussed in greater depth elsewhere in this issue.
Tinnitus may occur as a result of direct or, more frequently, indirect effects on the auditory system. It can be classified into the pulsatile and nonpulsatile varieties. Pulsatile tinnitus is often the result of nonlaminar flow and among other disease may arise from vascular or neoplastic causes, the latter of which is often based on microvascular shunting. Nonpulsatile tinnitus can result from dysfunction at any point along the ascending auditory system, often as a result of lesions that also cause hearing loss.
Within the external auditory canal and middle ear, conditions such as otitis, stapedius or tensor tympani myoclonus, and a variety of middle ear masses may cause tinnitus. In the bony labyrinth, conditions such as otosclerosis and Paget disease can be causative, which may be due to mechanical effects or intraosseous arteriovenous shunting. Within the membranous labyrinth, conditions such as Meniere disease or neoplasms such as endolymphatic sac tumors (ELSTs) may directly involve the endolymphatic system. A variety of medications may directly affect the cochlear hair cells but can also have more central toxicity in producing tinnitus. Some of the more common causes of pulsatile tinnitus may result from altered cerebrospinal fluid (CSF) pulsations and bone conduction transmitted to the cochlea, as suspected in idiopathic intracranial hypertension (IIH) and several vascular diseases. At the level of the internal auditory canal (IAC) and cerebellopontine angle (CPA) cistern, vestibular schwannomas are a frequently implicated culprit. Vascular loops may also contact the vestibulocochlear nerve within the IAC or at the level of the cisternal segment and, in some people, elicit tinnitus. Other multifaceted diseases can affect the vestibulocochlear nerve, including Chiari 1 malformations in which inferior brainstem descent may stretch the vestibulocochlear nerve and lead to tinnitus. A variety of intraparenchymal insults such as microangiopathic and demyelinating diseases may cause tinnitus, including those within the hindbrain in which lesions involving the Guillain-Mollaret triangle may manifest indirectly via myoclonus. More recently, numerous studies have shown evidence of functional abnormalities within the midbrain, thalamus, and various regions of the cerebral cortex, including the auditory cortex, as being responsible for tinnitus in the absence of findings on conventional imaging. Given its prevalence, tinnitus is a frequently encountered condition. Unfortunately, even after extensive work-up, a diagnosis is not discernible in approximately 60% of tinnitus sufferers. This highlights the need to maximize diagnostic yield, which begins by clinical stratification of the patients and often necessitates a multidisciplinary approach. Careful consideration of the explicit character of the tinnitus, attention to precipitating factors, and the presence of concomitant signs and symptoms can help guide the workup. In addition, a neurotologic examination is a must in the work-up of tinnitus. Tinnitus is principally categorized as either pulsatile, which can be subjective or objective, or nonpulsatile, which is almost always subjective. When possible, pulsatile tinnitus symptoms should be further subdivided into arterial causes, which are often synchronous with the heartbeat, and venous causes which are sometimes alleviated by compression of the internal jugular vein. For a detailed discussion on the clinical evaluation of tinnitus, (See Hertzano R, Teplitzky TB, Eisenman DJ: Clinical evaluation of tinnitus , in this issue.)
Background (epidemiology, pathophysiology, and anatomy: clinical perspective)
Tinnitus can be broadly defined as an auditory perception of internal origin. It represents a variety of aural sensations, including high or low frequencies that can be constant or intermittent in character. Tinnitus is not a diagnosis but a symptom with a potpourri of possible causes and correspondingly divergent pathophysiologic, anatomic, diagnostic, and therapeutic considerations. Epidemiologic studies have produced disparate chronic tinnitus prevalence estimates mostly in the range of 8% to 20% in the Western world, the variability likely owing to the heterogeneity of tinnitus and inconsistency with respect to methodology. Although it can present at any point in life, its incidence increases with age as well as in the presence of a variety of comorbidities and other symptoms, including hearing loss. It can be a source of persistent angst in a subset of patients and in 20% of tinnitus sufferers the severity is such that their quality of life is significantly impaired. This article aims to provide a summary of the imaging findings of structural causes of tinnitus, several of which are discussed in greater depth elsewhere in this issue.
Tinnitus may occur as a result of direct or, more frequently, indirect effects on the auditory system. It can be classified into the pulsatile and nonpulsatile varieties. Pulsatile tinnitus is often the result of nonlaminar flow and among other disease may arise from vascular or neoplastic causes, the latter of which is often based on microvascular shunting. Nonpulsatile tinnitus can result from dysfunction at any point along the ascending auditory system, often as a result of lesions that also cause hearing loss.
Within the external auditory canal and middle ear, conditions such as otitis, stapedius or tensor tympani myoclonus, and a variety of middle ear masses may cause tinnitus. In the bony labyrinth, conditions such as otosclerosis and Paget disease can be causative, which may be due to mechanical effects or intraosseous arteriovenous shunting. Within the membranous labyrinth, conditions such as Meniere disease or neoplasms such as endolymphatic sac tumors (ELSTs) may directly involve the endolymphatic system. A variety of medications may directly affect the cochlear hair cells but can also have more central toxicity in producing tinnitus. Some of the more common causes of pulsatile tinnitus may result from altered cerebrospinal fluid (CSF) pulsations and bone conduction transmitted to the cochlea, as suspected in idiopathic intracranial hypertension (IIH) and several vascular diseases. At the level of the internal auditory canal (IAC) and cerebellopontine angle (CPA) cistern, vestibular schwannomas are a frequently implicated culprit. Vascular loops may also contact the vestibulocochlear nerve within the IAC or at the level of the cisternal segment and, in some people, elicit tinnitus. Other multifaceted diseases can affect the vestibulocochlear nerve, including Chiari 1 malformations in which inferior brainstem descent may stretch the vestibulocochlear nerve and lead to tinnitus. A variety of intraparenchymal insults such as microangiopathic and demyelinating diseases may cause tinnitus, including those within the hindbrain in which lesions involving the Guillain-Mollaret triangle may manifest indirectly via myoclonus. More recently, numerous studies have shown evidence of functional abnormalities within the midbrain, thalamus, and various regions of the cerebral cortex, including the auditory cortex, as being responsible for tinnitus in the absence of findings on conventional imaging. Given its prevalence, tinnitus is a frequently encountered condition. Unfortunately, even after extensive work-up, a diagnosis is not discernible in approximately 60% of tinnitus sufferers. This highlights the need to maximize diagnostic yield, which begins by clinical stratification of the patients and often necessitates a multidisciplinary approach. Careful consideration of the explicit character of the tinnitus, attention to precipitating factors, and the presence of concomitant signs and symptoms can help guide the workup. In addition, a neurotologic examination is a must in the work-up of tinnitus. Tinnitus is principally categorized as either pulsatile, which can be subjective or objective, or nonpulsatile, which is almost always subjective. When possible, pulsatile tinnitus symptoms should be further subdivided into arterial causes, which are often synchronous with the heartbeat, and venous causes which are sometimes alleviated by compression of the internal jugular vein. For a detailed discussion on the clinical evaluation of tinnitus, (See Hertzano R, Teplitzky TB, Eisenman DJ: Clinical evaluation of tinnitus , in this issue.)
Imaging approach
Imaging Perspective
Pulsatile tinnitus has a broad range of reported imaging yield with most published estimates in the range of 57% to 100%. Conversely, almost all patients with nonpulsatile tinnitus do not have imaging abnormalities. Further complicating the picture, a diversity of lesions and anatomic variants that can be responsible for either type of tinnitus are also seen in the imaging of other patients in the absence of tinnitus. This last consideration may, in fact, be responsible for the variability in the published imaging yield.
There are multitudes of tinnitus imaging algorithms that have been advocated and published but currently there is no broadly accepted expert consensus. There are in fact multiple ways to arrive at a correct diagnosis in the imaging of tinnitus and the various modalities are often complementary, with a general algorithm proposed in Fig. 1 . Cost and patient safety are important considerations but the manner in which the imaging workup proceeds may frequently be based on institutional preference or availability and thus the authors advocate some general guidelines.
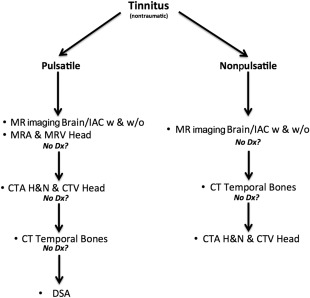
Imaging Guidelines
In nonpulsatile tinnitus, the diagnosis of IAC or CPA cistern masses may be the most significant pretest considerations and their presence is best assessed with MR imaging IAC protocol with and without contrast. As an example, the authors’ institution’s protocol is detailed in Table 1 .
| Precontrast | Postcontrast (half-dose, weight-based) |
|---|---|
| Trace-ADC-diffusion whole brain axial 5 mm | T1 fat saturated IAC axial 3 mm |
| FLAIR Whole Brain Axial 5 mm | T1 IAC coronal 3 mm |
| a CISS IAC 1 mm | b Radial VIBE IAC 2 mm |
| — | T1 whole brain axial 5 mm |
a 3D-stimulated T2 gradient-echo siemens sequence.
Similarly, most instances of pulsatile tinnitus merit assessment with MR imaging IAC with and without contrast. A typical MR imaging protocol is described in Table 1 . In addition intracranial MR angiogram (MRA) and MR venogram (MRV) provide significant complementary information and, therefore, are recommended in the imaging work-up. Some institutions suggest use of contemporaneous time-resolved (4-dimensional) MRA sequences allowing for noninvasive dynamic assessment with a temporal resolution in the range of 1 millisecond and elucidation of a variety of vascular diseases not readily discernible on routine imaging. An alternative approach, especially if a suspicion for venous tinnitus and idiopathic intracranial hypertension exists, may be to begin with a CT of the temporal bones and a CT venogram (See Michael A. Reardon MA, Raghavan P: Venous abnormalities leading to tinnitus-imaging evaluation , in this issue). Should these be negative, CT angiogram (CTA) of the head and neck and Doppler carotid sonography are reasonable noninvasive adjuncts to look for causes such as atherosclerotic stenosis, among other causes. Digital subtraction angiography (DSA) can often be reserved as a tertiary modality to fully exclude small vascular malformations. The imaging workup and the radiologist’s approach to interpreting imaging studies in patients with tinnitus must, therefore, be guided by an understanding of the clinical scenario. If there is loud, objective pulsatile tinnitus or other strong clinical suspicion for a vascular malformation, then consideration could be given to DSA as an earlier, more definitive option and to potentially allow for prompt endovascular treatment. In the setting of an abnormal otoscopic examination suggesting a middle ear mass or evidence for osseous disease, a high-resolution temporal bone CT would be the more appropriate first study. In the setting of acute onset of symptoms, particularly with a history of trauma, other severe conditions, such as carotid dissection, should be considered and may be initially worked up by a CTA of the head and neck, which is often readily available. Other imaging studies, such as temporomandibular joint MR imaging, are uncommonly performed but can be used in appropriate clinical scenarios when other studies do not provide any diagnostic information. Knowledge of the clinical situation also helps determine the search pattern while examining these imaging studies. For example, a history of pulse synchronous tinnitus, obliterated by pressure on the internal jugular vein should prompt close scrutiny of the sigmoid sinus wall for dehiscences and diverticula and of the brain and orbits for signs of intracranial hypertension. A history of a reddish mass in the middle ear on otoscopy should guide a search for paragangliomas, jugular bulb abnormalities, and otosclerosis, and warrants close attention to the mesotympanum and hypotympanum, the jugular foramen, and bony labyrinth. It is important to remember that dural arteriovenous fistulae may not be immediately apparent on cross-sectional imaging and subtle signs such as asymmetrically prominent vessels and irregularity of venous sinus walls, for example, must be carefully sought. Some advanced imaging techniques such as functional MR imaging have provided insight in the research of tinnitus and may eventually find a role in the clinical realm. For a discussion on the role of advanced imaging techniques in tinnitus, (See Raghavan P, Steven A, Gandhi D: Advanced neuroimaging of tinnitus , in this issue.)
Differential diagnosis
An exhaustive discussion of the myriad causes that can cause tinnitus is impractical. This article, therefore, focuses on some of the more common causes and a few unusual but important causes responsible for tinnitus. Given the realities of imaging yield (see previous discussion), the focus will inherently trend toward diseases more frequently associated with pulsatile tinnitus but we will also highlight some causes more typically associated with nonpulsatile tinnitus.
Vascular causes
Vascular Malformations
Arteriovenous fistulae (AVF) and arteriovenous malformations (AVMs) are common causes of pulsatile tinnitus. In fact, they are the most common causes in patients with objective pulsatile tinnitus and a normal otoscopic examination.
Arteriovenous fistulae
AVF are characterized by an anomalous direct communication between an artery and vein without interposed vascular nidus. They are thought to be acquired and can be the sequela of trauma, infection, or venous sinus thrombosis. Dural AVF are most frequently supplied by branches of the external carotid artery, and those draining via the transverse and sigmoid sinuses may be the most common ones to cause tinnitus. However, other AVF may be implicated, including those with cortical venous or leptomeningeal drainage, cavernous carotid fistulae, and even those within the scalp, face, and neck. MR imaging findings in AVF can include prominent flow voids, with associated intracranial hemorrhage and/or intraparenchymal hyperintensity (ie, edema, infarction, or gliosis).
Arteriovenous malformations
AVMs are usually pial-based lesions characterized by an abnormal connection between artery and vein with interposed vascular nidus. There are several subtypes based on differences in angioarchitecture and location, any of which may present as tinnitus. They are generally thought to be congenital; however, they most frequently present in the third or fourth decades of life. Intracranial AVMs are usually supplied by branches of the internal carotid arteries or the vertebrobasilar system. MR imaging findings in intra-axial AVM include a tangle of flow voids (so-called bag of worms) most frequently located in the cerebral hemispheres. AVMs carry an approximately 4% annual risk of hemorrhage but a variety of characteristics may alter their course. While evaluating the imaging features of these lesions, the radiologist should vigilantly assess for the presence of findings that may herald a more aggressive clinical course, such as flow-related or intranidal aneurysms, and a pattern of diffuse leptomeningeal vein prominence, the so-called pseudophlebitic pattern.
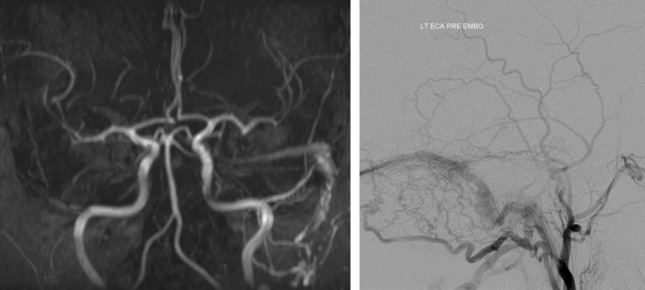
MRA using dynamic sequences will show arteriovenous shunting as early filling veins in most of these vascular malformations and can provide critical details about lesion character and complexity for classification and treatment planning, most closely rivaling the diagnostic capabilities of DSA. Despite the utility of these modern MRA techniques, DSA remains the gold standard in the setting of vascular malformations and allows for endovascular treatment when appropriate ( Fig. 2 ).
Acquired Arterial Diseases
Aneurysms
Aneurysms can occasionally present as pulsatile tinnitus, most notably those arising from the petrous carotid artery. It is theorized that this may be in part due to compressive obliteration of the adjacent venous plexus that may normally act to dampen the carotid pulsations. With the loss of this buffer, the pulsations within the aneurysm may then be transmitted either directly or via a bone conduction mechanism to the cochlea. However, other mechanisms can contribute. For example, in the setting of aneurysm rupture, hemorrhage itself can result in acute tinnitus as 1 of several symptoms. On an MR imaging study, a petrous carotid aneurysm can be confused with a variety of petrous apex mass lesions. However, a CTA should provide unequivocal evidence for the correct diagnosis ( Fig. 3 ).
Arterial dissection
Arterial dissection can be seen in multiple settings, including trauma, and may occasionally produce pulsatile tinnitus, particularly when involving the extracranial internal carotid arteries. When present, additional symptoms of cerebral ischemia or Horner syndrome will typically coexist with tinnitus. If clinically suspected, angiographic evaluation should be promptly undertaken via CTA, DSA, or MRA to facilitate treatment initiation. The most frequent finding on both CTA and time-of-flight MRA is smooth, tapered (flame-shaped) narrowing of the vessel lumen. When available, an MRA may be the best modality and should also include a fat suppressed axial T1 weighted (T1W) sequence through the neck ( Fig. 4 ) as well as a diffusion-weighted sequence of the brain. The former allows evaluation for a potentially compressive intramural thrombus or hematoma that will appear as a hyperintense crescent, whereas the latter allows evaluation for presence of ischemic infarcts, which is an important factor when considering anticoagulation.
Atherosclerotic stenosis
Atherosclerotic stenosis, particularly involving the extracranial carotid arteries, has been described as the second-most frequent cause of pulsatile tinnitus in at least 1 study. Interestingly, the laterality and degree of stenosis do not always correlate with the tinnitus symptoms and the cause of the tinnitus in these patients may be a direct result of turbulent flow, an indirect effect of sympathetic activation or some combination thereof. However, atherosclerotic carotid stenosis treated with endarterectomy has been shown to relieve tinnitus symptoms in some patients. Subclavian steal phenomenon is most frequently due to atherosclerotic stenosis at the subclavian artery origin and can also present with tinnitus. The mechanism of tinnitus in this setting is purportedly related to anastomoses between the vertebral artery and the occipital artery. CTA has several advantages compared with DSA, MRA, and ultrasound in these settings. Specifically, CTA offers high spatial resolution, allows for accurate discrimination of atherosclerotic plaque composition (ie, soft or calcific), and fosters elucidation of coexistent diseases such as ulceration and dissection. However, Doppler carotid sonography avoids exposure to ionizing radiation and may also be the most cost-effective and efficient modality for evaluation of the neck vessels. Ultrasound can depict turbulent flow via spectral broadening while accurately grading most stenoses via peak systolic velocity measurement. In suspected subclavian steal, both sonography and MRA can reliably show flow reversal in the vertebral artery, which is not feasible by conventional CTA.
Fibromuscular dysplasia
Fibromuscular dysplasia is an idiopathic angiopathy that affects medium-sized arteries and is seen most frequently in young to middle-aged women. Cerebrovascular involvement, particularly of the carotid arteries, is not infrequent in this condition, being second only to the renal arteries. With carotid involvement, tinnitus is the second-most common presenting symptom after cerebral ischemic symptoms and is seen in approximately 30% of patients. As with carotid stenosis, the mechanism of the symptoms may be some combination of several factors and may be further exacerbated by the frequent coexistence of renovascular hypertension in these patients. Whether seen on carotid sonography or any angiographic study, the classic pathognomonic appearance of fibromuscular dysplasia is the string-of-beads sign, reflecting the segmental corrugated morphology of the vessels ( Fig. 5 ). However, it may also present with stenosis, typically high in the cervical segment internal carotid artery. Potential cerebrovascular sequelae for which the radiologist must remain alert include aneurysms (seen in 30% of cases), spontaneous dissections (seen in 10%–20%), and focal ischemic infarcts. Thus, brain MR imaging and intracranial MRA are also highly recommended in these patients.






