- •
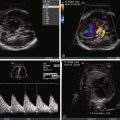
Trace and identify the insertion of the umbilical vein into the fetus and follow its course to the heart.
- •
Determine whether the umbilical vein connects to the liver (i.e., portal or hepatic venous system) or connects extrahepatically (i.e., to a systemic vein or coronary sinus or directly to the heart).
- •
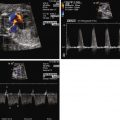
Assess for heart size via the cardiothoracic ratio.
- •
Assess for systolic function.
- •
Assess for the presence and grade of atrioventricular valve regurgitation.
- •
Look for signs of pleural effusion, pericardial effusion, or hydrops as a consequence of heart failure due to volume overload.
- •
Determination of combined cardiac output can be helpful in serial evaluation.
Anatomy and Anatomical Associations
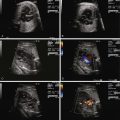
The ductus venosus (DV) is a short, hourglass-shaped venous structure that shunts umbilical venous return to the fetal heart. It is part of a collection of vessels located beneath the diaphragm that is involved in carrying placental venous return back to the fetus. The anatomical arrangement of venous return from the placenta is designed to optimize streaming of flow of the most highly oxygenated blood to the most essential developing organs. A review of the venous anatomy leading from the placenta to the fetal heart is important in order to understand the implications of absence or agenesis of the DV.
Figure 37-1 demonstrates this unique and complex anatomy. Venous return from the placenta to the fetus can be conceptualized with the liver as the primary focal point. There are (1) afferent veins—the umbilical and portal veins and (2) efferent veins—the hepatic veins and subdiaphragmatic vestibulum, going toward and emerging from the liver, respectively.

The umbilical vein enters the fetal abdomen within the falciform ligament and ascends steeply toward the liver where it runs along its inferior surface. The umbilical vein then joins a confluence of vessels termed the portal sinus. The portal sinus is a vascular space and is a conglomeration of structures including (1) the intrahepatic portal vein, made up of the left and right portal veins, (2) the extrahepatic portal vein comprising the splenic and mesenteric veins, and (3) the DV. Blood flows into the portal sinus from the umbilical vein and is then dispersed into the liver via the afferent system of the portal veins. Blood exits the liver parenchyma via the efferent system of hepatic veins, which coalesce into a subdiaphragmatic vestibulum or chamber together with the inferior vena cava, just prior to entry into the right atrium.
Because the umbilical vein is closer to the left portal vein than to the right, the left portal vein and left lobe of the liver receive relatively highly oxygenated blood. The extrahepatic portal vein carries poorly oxygenated blood and it enters closer to the right portal vein. Oxygen consumption within the liver is relatively low, and as the blood entering the left lobe of the liver is preferentially streamed from the umbilical vein, blood exiting the left hepatic vein is relatively highly oxygenated in comparison with the right hepatic vein.
The DV can be thought of as a structure that acts as a bypass, or shunt, between the afferent and the efferent venous systems. The DV originates from the superior aspect of the portal sinus and constricts slightly as it heads cephalad. An important point to recognize is that there is no anatomical continuity between the umbilical vein and the DV, but in fact, the latter originates from the portal sinus. However, its ostium is aligned with the outlet of the umbilical vein; hence, it carries richly oxygenated blood. The DV has a pinched appearance and is slightly constricted relative to the proximal portal sinus and umbilical vein. This acts to increase kinetic energy and accelerate flow, thereby directing a stream of highly oxygenated blood through the subdiaphragmatic vestibulum angled toward the foramen ovale. There is debate as to whether or not a muscular sphincter is associated with the DV. Histopathological analysis demonstrates the presence of endothelial corrugations and innervated smooth muscle, which supports the hypothesis that the DV is an actively regulated vessel with the capacity to rapidly change diameter along its entire length in response to certain stimuli. The left hepatic vein also carries highly oxygenated blood and runs parallel with the DV, whereas the right hepatic vein runs parallel with the inferior vena cava. Hence, left hepatic venous effluent also contributes somewhat to the preferential streaming of oxygenated blood to the foramen ovale and left side of the heart. Color Doppler studies have shown an interesting symmetry in that the left-sided DV and left portal vein axis carries relatively highly oxygenated blood, and the inferior vena cava and right portal vein axis carries relatively poorly oxygenated blood that is directed more rightward into the right atrium.
Perhaps somewhat underappreciated is the fact that the DV does not carry the full complement of umbilical venous return. Placental venous return is shared by the DV and the portal-hepatic venous system. In essence, the liver and the DV are partners in guarding placental venous return to the fetus. One function of the DV is to divert umbilical venous blood away from the liver. The DV can be considered a physiological “resistance valve”—when tone is high, blood is shunted into the liver; when tone is relaxed, more blood passes through it directed toward the foramen ovale. It is believed that, early in gestation, up to 50% of the umbilical venous return is shunted through the DV, which decreases to 20% to 30% in the latter half of the third trimester. The amount of blood shunted through the DV can change under conditions of stress. During periods of hypoxemia or placental insufficiency, a greater proportion of umbilical venous return is shunted through the DV. This appears to be an adaptive mechanism aimed at preserving preferential streaming and delivery of richly oxygenated blood to the left side of the heart at times of greatest need.
A substantial amount of blood is directed through the liver before it reaches the fetal heart; the proportion directed into the liver increases with gestational age. The significance of this phenomenon, particularly in terms of development of the liver—or conversely and perhaps more important, the contribution of the liver to overall fetal development by release of biologically active elements into its venous effluent—is still unclear and worthy of investigation.
Absence, or agenesis, of the DV results in two general alternate pathways of umbilical venous return: (1) intrahepatic, in which the umbilical vein connects to the portal system, usually the left portal vein, or (2) extrahepatic, in which the umbilical vein connects to a venous structure outside of the liver such as the femoral veins, iliac veins, inferior vena cava, coronary sinus, or atria. Regardless of the type, agenesis of the DV results in a loss of the preferential streaming phenomenon of umbilical venous return across the foramen ovale to the left heart. In the intrahepatic type, umbilical venous return is still “washed” through the liver and blood must make its way through the hepatic microcirculation before ending up in the heart. The hepatic vasculature then acts as a “resistor” to umbilical venous return, in a similar fashion to the DV. Absence of such a resistor can result in unimpeded umbilical venous return and a potential torrent of placental blood flow flooding the fetal circulation. Such is the case when the umbilical vein connects in an extrahepatic manner.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree