- •
Patency of the tricuspid valve
- •
Size of the right ventricle
- •
Size and location of the ventricular septal defect, which directs blood into the right ventricle
- •
Relationship of the great vessels, as normally related or transposed
- •
Size of the main and branch pulmonary arteries (normally related great vessels)
- •
Size of the aorta and aortic arch (transposed great vessels)
- •
Doppler assessment of the presence or absence of antegrade flow across the pulmonary valve
- •
Doppler assessment of antegrade or retrograde flow across the patent ductus arteriosus
- •
Left ventricle size, geometry, and function
Anatomy and Anatomical Associations
Tricuspid atresia is an anomaly in which the tricuspid valve is fully sealed off without any flow across. There are four morphologic types of tricuspid atresia: muscular (62%), membranous (29%), Ebstein-like (6%), and valvar (3%). In muscular tricuspid atresia, no valve is present and muscle lines the floor of the atrium. In membranous tricuspid atresia, the membranous atrioventricular septum lines the floor of the atrium. In Ebstein-like tricuspid atresia, the cavity of the right ventricle (RV) is lined with imperforate valve tissue that is displaced apically, with “atrialization” of the RV. Finally, in valvar tricuspid atresia, valve tissue and chordae are visible, although the valve is simply imperforate and sealed. RV hypoplasia is typically seen with tricuspid atresia as well as a ventricular septal defect (VSD). The severity of hypoplasia depends upon the size of the VSD. The inlet portion of the RV is absent because development of the tricuspid valve is related to the inflow (sinus) portion. Thus, in tricuspid atresia, the RV comprises the infundibular and trabecular portions. The branch pulmonary arteries are typically well formed and are perfused by a ductus arteriosus; however, there are cases of associated pulmonary artery hypoplasia.
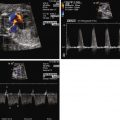
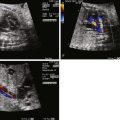
Tricuspid atresia is classified according to the relationship of the great arteries. In type I tricuspid atresia, which accounts for approximately 70% to 80% of all patients, there are normally related great arteries, Van Praagh segments {S,D,S}. In this type, there is effective pulmonary stenosis because flow is from the left ventricle across the VSD into the RV then into the pulmonary arteries. The size of the VSD dictates the degree of antegrade pulmonary blood flow into the main pulmonary artery ( Figures 27-1 and 27-2 ). In type II tricuspid atresia, which accounts for approximately 12% to 25% of cases, there are transposed great arteries ( d -transposition of the great arteries, segments {S,D,D}). With transposed great arteries, the VSD leads to the RV, which gives rise to the aorta and systemic outflow tract, leading to variable degrees of subaortic stenosis and/or coarctation of the aorta ( Figure 27-3 ). Type III is relatively uncommon, accounting for approximately 3% to 6% of patients, and refers to patients with more complex lesions, such as l -transposition or corrected transposition of the great arteries.



The foramen ovale in tricuspid atresia plays an essential role, because all of the venous return and, hence, cardiac output must travel across it from right to left. In types I and II, the patent foramen ovale is usually large and unrestrictive. In type III patients with l -looped ventricles, the patent foramen ovale may become restrictive over the course of gestation, because the tricuspid valve is then on the left side and guards the chamber receiving pulmonary venous return.
Tricuspid atresia usually occurs as an isolated defect. However, less than 20% of all patients have associated defects. An atrial septal defect is the most commonly seen anomaly. Approximately 8% of patients with tricuspid atresia have coarctation of the aorta, specifically those with transposed great vessels. Other associated cardiac anomalies include left superior vena cava, left juxtaposition of the atrial appendages, and right aortic arch.
Frequency, Genetics, and Development
Tricuspid atresia accounts for 1% to 3% of congenital heart disease. In one study, the prevalence was 0.057 cases/1000 live births. Most cases of tricuspid atresia are sporadic, although families with multiple affected members have been reported. The cause of tricuspid atresia is currently not known, although tricuspid atresia has been reported in mice with Fog-2 or Hey 2 mutations, two transcription factors involved in cardiac morphogenesis. Although rare, tricuspid atresia has been reported with 22q11 deletion.
Prenatal Physiology
In the fetus with type I or type II tricuspid atresia, there is obligatory right-to-left shunting across the patent foramen ovale. As a consequence, the patent foramen ovale is typically large and unrestrictive. Increased flow to the left ventricle results in left ventricular dilation and hypertrophy. Restriction at the level of the patent foramen ovale is unusual, although reversal with atrial contraction at the ductus venosus or even venous pulsations may be seen if the patent foramen ovale becomes restrictive. Hydrops fetalis is not typically seen, except in the rare setting of a restrictive patent foramen ovale, which is life-threatening to the fetus.
With normally related great arteries (type I tricuspid atresia), the size of the VSD determines the amount of antegrade pulmonary blood flow. Because the VSD is the proximal controller of blood flow into the RV, hypoplasia of the pulmonary annulus or pulmonary valve stenosis can develop if there is limited flow across the VSD. In its most severe form, with an intact ventricular septum, there is effective pulmonary atresia. Flow in the ductus arteriosus is reversed. With a small or restrictive VSD, antegrade blood flow is limited; there may or may not be flow reversal in the ductus arteriosus. With a large or unrestrictive VSD, there is no reversal of flow in the ductus arteriosus because there is an abundance of antegrade blood flow into the RV. The VSD is typically classified as a perimembranous defect, although muscular VSDs, which may become more restrictive over the course of gestation, may also be seen. Multiple VSDs are also possible.
With transposed great vessels (type II tricuspid atresia), the size of the VSD determines whether or not there is any obstruction to systemic outflow. Consequently, fetuses with tricuspid atresia and transposed great vessels are at risk for subaortic obstruction, aortic hypoplasia, and coarctation of the aorta. With significant aortic arch obstruction, the ductus arteriosus is large and supports the systemic perfusion and there may flow reversal seen in the transverse arch to perfuse the head and neck vessels.
In type III tricuspid atresia with l -looped ventricles, restriction at the level of the patent foramen ovale is more common than in Type I or II. Consequently, with obstruction to pulmonary venous egress, these fetuses are at greater risk for pulmonary hypertension postnatally secondary to maldevelopment of the pulmonary vascular bed.
Prenatal Management
Most fetuses with tricuspid atresia fare well over the course of gestation. In a recent series of 88 fetuses prenatally diagnosed with tricuspid atresia, there were 58 live-born, 4 with in utero demise, 25 terminations of pregnancy, and 1 lost to follow-up. In the absence of hydrops fetalis, fetuses with tricuspid atresia tolerate a vaginal delivery well. Those with restrictive interatrial communications are at risk for development of hydrops fetalis; however, this is a relatively rare phenomenon. In addition, mitral regurgitation and left ventricular dysfunction may progress over the course of gestation, predisposing the fetus to the development of hydrops fetalis.
Diagnosis of tricuspid atresia on fetal imaging is made by assessment of the four-chamber view. On two-dimensional imaging, there is absence of an opening at the site of the tricuspid valve and there is associated right ventricular hypoplasia. Color Doppler imaging will display absence of flow across the region of the tricuspid valve. Assessment of VSD and RV size can be made in multiple planes. The size of VSD and pulmonary annulus, and assessment of the degree of antegrade flow across the pulmonary valve, will dictate whether there will be adequate pulmonary blood flow at birth or the need for prostaglandin E (PGE) infusion to maintain ductal patency as a source of supplemental pulmonary blood flow. If the ductus arteriosus flow in utero is all antegrade, this suggests an adequate size pathway for antegrade pulmonary blood flow. If there is reversal of flow in the ductus arteriosus (aorta to pulmonary artery), it becomes more difficult to judge before birth, and judgment must await postnatal evaluation.
The VSD may become more restrictive over the course of gestation, leading to more severe pulmonary outflow tract obstruction in the case of normally related great arteries or aortic outflow tract obstruction in the case of transposed great arteries. Hence, serial evaluation is called for. In our practice, we evaluate such patients every 4 weeks to reassess the anatomy as well as to continue educational and supportive activities with the family.
There is currently no accepted way to rehabilitate the RV with tricuspid atresia and perform a two-ventricle repair; hence, management involves a single-ventricle palliation. However, not all fetuses with tricuspid atresia require intervention in the neonatal period. In fetuses with pulmonary atresia or severe pulmonary stenosis with flow reversal in the ductus arteriosus, prostaglandin should be initiated at birth to support pulmonary blood flow with plans for a systemic–to–pulmonary artery shunt in the neonatal period. In fetuses with tricuspid atresia, transposed great vessels, and a suspected coarctation of the aorta, prostaglandin is initiated to maintain ductal patency in support of systemic circulation in anticipation of a stage 1 Norwood procedure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree