- •
Size and function of the right ventricle in comparison with the left ventricle.
- •
Morphology of the right ventricle (i.e., tripartite?).
- •
Morphology of the pulmonary outflow tract.
- •
Patency of the pulmonary valve.
- •
Tricuspid valve size and morphology.
- •
Presence or absence of tricuspid regurgitation.
- •
If tricuspid regurgitation is present, estimate the intracavitary right ventricular pressure by Doppler estimate of the peak velocity of the regurgitant jet.
- •
Presence or absence of right ventricle coronary-cameral fistulas.
- •
Presence or absence of coronary artery dilation along the septum or surface of the heart.
- •
Presence or absence of left ventricular outflow tract obstruction (i.e., septal bowing).
Anatomy and Anatomical Associations
Pulmonary atresia with intact ventricular septum (PA/IVS) is an anomaly in which the pathway from the right ventricle (RV) to the pulmonary artery is completely sealed off (PA) and there is no ventricular septal defect. PA/IVS is a morphologically heterogeneous disorder. The RV is commonly small or hypoplastic, or it can be markedly dilated, as when associated with Ebstein’s anomaly or tricuspid valve (TV) dysplasia and severe tricuspid regurgitation. There may be muscular atresia of the right ventricular infundibulum with no outflow chamber at all or there may be platelike valvar atresia. In addition, PA may be acquired over the course of gestation. Critical pulmonary stenosis may progress to PA. In addition, decreased forward flow through the right ventricular outflow tract in the setting of severe tricuspid regurgitation, as in Ebstein’s anomaly or the twin-twin transfusion syndrome, may lead to “functional” PA in which the valve leaflets do not open based on hemodynamics but, anatomically, do have the potential to open. Such cases of “functional” atresia can progress to true, anatomical PA. At birth, the pulmonary valve leaflets may be fused and appear clinically indistinguishable from congenital forms of PA.
Extracardiac anomalies are unusual in PA/IVS, although many different cardiac anomalies can be seen. Atrial septal defects and abnormalities of the right side of the heart are common. In PA/IVS, the TV leaflets are frequently dysplastic. The chordae of the TV may be thickened and may not adequately coapt, causing severe regurgitation in utero. In addition, in many cases, the TV annulus is small. It may be moderately to severely hypoplastic, causing tricuspid stenosis or, in some cases, fully seal off and manifest tricuspid atresia.
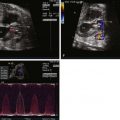
The morphology of the RV may be abnormal and is an important component of the anomaly. The RV is anatomically considered to consist of three parts (tripartite). It can be divided into (1) an inlet or sinus portion, which is the region just beneath the TV, (2) a trabecular or muscular portion, which makes up the apex, and (3) an outlet portion, which consists of the infundibulum. In PA/IVS, there may be severe underdevelopment and distortion of the ventricle so that only an inlet portion is recognized ( Figure 25-1 ). Interestingly, pulmonary arterial abnormalities are rare in PA/IVS. The branch pulmonary arteries are typically of good size and perfused by the ductus arteriosus.
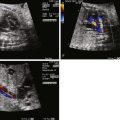
Coronary abnormalities are common in PA/IVS, especially in the setting of a hypertrophied, hypertensive RV with little or no tricuspid regurgitation. There is an inverse relationship between RV size and coronary abnormalities—the smaller the RV and tricuspid annulus, the more likely there will be coronary abnormalities. There may be atresia of the coronary os from the aortic root, hemodynamically significant coronary stenoses, or even coronary interruptions. In these cases, coronary arterial flow depends upon perfusion from the RV through connections into the right ventricular cavity called right ventricular sinusoids or fistulas. If a portion of the myocardium is solely dependent upon coronary perfusion from the RV alone, there is “RV-dependent coronary circulation.”
The prevalence of coronary arterial abnormalities in PA/IVS is as follows. Fistulous communications between the RV and the coronary arteries are noted in 75% of patients. Of these, 34% have coronary interruptions, 16% have atresia of the aortocoronary os, and 5% have a single coronary orifice. In one study, 40% of patients were deemed to have RV-dependent coronary arterial circulation. Myocardial abnormalities may be seen in conjunction in PA/IVS. These abnormalities include myocardial disarray, spongy myocardium, and ventricular endocardial fibroelastosis. In the setting of coronary abnormalities, ischemia, fibrosis, infarction, and myocardial rupture have been observed in fetuses with PA/IVS.
Finally, left heart abnormalities may be seen in PA/IVS. Left ventricular outflow tract obstruction may occur at the subvalvar and valvar levels. In cases of suprasystemic right ventricular pressure with severe septal hypertrophy, the septum may bow right to left into the left ventricular outflow tract causing subaortic obstruction. In utero infarction of the ventricular septum can cause septal aneurysm and distortion, leading to left ventricular outflow obstruction.
Frequency, Genetics, and Development
PA/IVS is a rare congenital heart defect with no known genetic etiology. The prevalence of PA ranges from 4.1 to 8.3 per 100,000 live births and accounts for approximately 3% of serious congenital heart defects. Males and females are affected equally. Multifactorial inheritance suggests a risk of recurrence of 3% to 5%.
The coronary arteries develop from the epicardial surface of the heart and normally migrate toward the aorta. In PA/IVS, normal coronary development is altered. The hypertensive RV may act as an “attractant,” directing coronary migration toward it. Alternatively, very early natural connections between the developing RV and the coronary circulation may persist in order to allow for decompression and a pathway out of the embryonic RV, when the pulmonary valve is atretic. Over time, as the RV develops further hypertension, these primitive RV-to-coronary connections are preserved, and competitive flow between the aorta and the RV lead to narrowing and dilation (stenosis and ectasia) at the connections.
Prenatal Physiology
In the fetus with PA/IVS, there is no antegrade flow across the pulmonary valve. Consequently, all flow across the ductus arteriosus is retrograde (aorta–to–pulmonary artery), supplying the branch pulmonary arteries. Because blood cannot effectively make its way across the right side of the heart, there is increased right-to-left shunting at the atrial communication, leading to relative dilation of the left side of the heart. Restriction at the atrial communication will impair venous return and may lead to development of hydrops fetalis. The size of the TV and the degree of tricuspid regurgitation dictate the course.
In cases of severe tricuspid regurgitation with a normal TV annulus, the right atrium and RV become dilated, placing the fetus at risk for arrhythmia and hydrops fetalis. Marked cardiomegaly with cardiothoracic area ratios greater than 0.60 may compromise pulmonary vascular and lung development. . In these, the prognosis is extremely poor. In cases of severe tricuspid stenosis with little tricuspid regurgitation, the RV is severely hypertrophied and noncompliant. Pressures within the hypoplastic right ventricular cavity may reach suprasystemic and fistulous communications between the RV and the coronary arteries may be present. These coronary sinusoids place the fetus at risk for myocardial ischemia, arrhythmia, and potential fetal demise.
Prenatal Management
At initial encounter of a fetus with PA/IVS, prognosis and counseling hinge on assessment of the anatomy as being most favorable for a two-ventricle (biventricular) repair strategy or a single-ventricle palliative strategy. In essence, the questions to be answered are, (1) Can the RV undertake the task of perfusing the pulmonary circulation? and if so, (2) How best to get there and achieve this goal? If deemed that the RV cannot be recruited to this task based on anatomical features that make it prohibitive, a single-ventricle palliative strategy is to be undertaken.
Many investigators have looked into identifying fetal predictors of the eventual postnatal circulation. Gardiner and coworkers published gestational age–specific criteria for predicting postnatal single-ventricle palliation versus biventricular repair. Predictive variables included the TV/mitral valve annular diameter ratio, Z-scores for the pulmonary valve (indexed to fetal size based upon femoral length), the right atrial “pressure score” (a composite based upon the TV Doppler, foramen ovale, and ductus venosus Doppler flow patterns), and the presence of coronary fistulas. Salvin and colleagues established the importance of the TV Z-score in the mid–gestational age fetus. In their study, all fetuses with a TV Z-score greater than −3 achieved a biventricular repair postnatally. Finally, Roman and associates established a scoring system utilizing four criteria for discriminating fetuses with PA/IVS likely to achieve a biventricular repair from a single-ventricle palliation. In their study, fetuses most likely to require a single-ventricle palliation had (1) TV/mitral valve annular diameter ratio less than 0.7, (2) RV/LV (left ventricle) length ratio less than 0.6, (3) TV inflow duration less than 32% of the cardiac cycle, and (4) fistulous connections to the RV.
Once the diagnosis is established, serial fetal echocardiography should be performed with focus on the features (see Key Features) as well as those in the previously discussed, published criteria in order to survey for findings that may change the prognosis from a two-ventricle to a single-ventricle strategy.
When coronary artery abnormalities are present in PA/IVS, it is important to distinguish between two categories of patients. In the first, there are connections between the RV and the coronaries (sinusoids or coronary-cameral fistulas) but dual supply from both the aorta and the RV, without any impediment to normal aorta-to-coronary perfusion. In the second, there are connections between the RV and the coronaries, but there is also impediment to aortic supply distal to the connection due to stenosis or atresia; hence, the distal coronary circulatory bed is dependent upon perfusion from the RV. This is referred to as an “RV-dependent coronary circulation.” In the first category, postnatal rehabilitation of the RV through relief of outflow obstruction and decompression is permissible, because coronary flow will continue via the aorta. However, in the second, such decompression will reduce RV pressure and eliminate the source for coronary perfusion resulting in myocardial ischemia. Distinguishing between these two categories using fetal echocardiography is not possible. Diagnosis of RV-dependent coronary circulation is best made using cardiac catheterization after birth.
Most fetuses with PA/IVS have no significant problems in utero. However, the presence of extensive fistulous communications places the fetus at risk for ischemia, arrhythmia, and sudden death in utero. Fistulous connections between the RV and the coronaries can be seen on fetal echocardiography. Color Doppler imaging scale should be lowered in order to increase the sensitivity of observing low-velocity flow. However, delineating details of coronary anatomy such as stenosis or source of perfusion is a challenge and should be left for postnatal assessment via cardiac catheterization.
Severe dilation of the right heart secondary to severe tricuspid regurgitation and massive cardiomegaly compromises lung development and may lead to hydrops fetalis. Impending hydrops fetalis may be an indication for early delivery via cesarean section. Fetal intervention for PA/IVS remains controversial, although proponents argue that fetal pulmonary valvuloplasty is indicated in cases of impending hydrops. In addition, in utero intervention may preserve the possibility for a biventricular repair postnatally. It can be said that, once specific features are seen, it is possible to determine which fetuses have an inadequate RV for a two-ventricle repair. However, predicting which fetuses with a current profile suggesting an adequate RV will go on during gestation to develop findings of an inadequate RV is a challenge. Such a path, and the prognostic echocardiographic features that would predict such, has not yet been described in the literature.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree