- •
Left ventricle dilation with poor contractility.
- •
Aortic annular hypoplasia, with aortic stenosis or atresia.
- •
Abnormal mitral valve with small annulus, thickened leaflets, stenosis, and severe regurgitation.
- •
Markedly dilated left atrium in the presence of severe mitral regurgitation.
- •
Highly restrictive or intact atrial septum.
- •
Abnormal Doppler flow pattern may be noted when sampling the pulmonary veins (“double-reversal” pulmonary vein flow pattern).
- •
Echo-bright left ventricle endocardium, reflecting endocardial fibroelastosis.
- •
Retrograde flow in the transverse and ascending aorta secondary to perfusion via the ductus arteriosus.
- •
Right ventricle may be compressed secondary to left-sided dilation.
- •
Hydrops fetalis possible when the right ventricle is compressed by the left ventricle.
Anatomy and Anatomical Associations
Mitral valve dysplasia syndrome (MVDS) is an uncommon form of congenital heart disease that is often thought to be synonymous with hypoplastic left heart syndrome (HLHS) or critical aortic stenosis. The anomaly is unique with specific characteristics and a constellation of findings that make this disorder deserving of recognition as a separate entity. Patients with MVDS share many anatomical and physiological similarities to those with HLHS, such as mitral stenosis and severe aortic stenosis/atresia leading to left ventricular outflow tract (LVOT) obstruction. In addition, they often have dilated, dysfunctional left ventricles, like patients with critical aortic stenosis. However, unique from those forms of left-sided obstructive heart disease, patients with MVDS have a primary abnormality of the mitral valve (MV). The MV is thick and dysplastic, resulting in significant mitral regurgitation (MR). In utero, these fetuses have marked left atrial dilation and restriction of the atrial septum. In addition, left ventricular dilation and dysfunction, often compromising right ventricular function, is characteristic of the disease. This combination of aortic outflow obstruction, severe MR, left heart dilation, and restriction of the atrial septum is what defines MVDS as a unique entity.
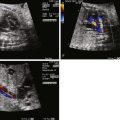
Pathological review of specimens with MVDS reveals the presence of a thick, dysplastic MV with absent chordae, characteristic of MV arcade ( Figure 23-1 ). In addition, they typically have severe aortic stenosis or atresia, with a monocuspid aortic valve. Left ventricle dilation and the presence of endocardial fibroelastosis (EFE) are common. The left atrium is thick-walled, with evidence of left atrial hypertension, and a restrictive or intact atrial septum is usually present.

MVDS is a rare constellation of cardiac findings, and to our knowledge, there are no other associated anatomic or extracardiac anomalies.
Frequency, Genetics, and Development
MVDS is a rare disorder. Because these patients have historically been categorized as having HLHS, the exact incidence is not known. In our institution, a review of our fetal database from 2002 to 2009 revealed 10 cases of MVDS diagnosed in utero. In addition, a handful of isolated case reports in the literature have described patients with this disease process, but no large case series has been reported.
The developmental progression of MVDS in the fetus may be similar to the development of HLHS. As hypothesized in HLHS, patients with MV dysplasia have aortic stenosis and decreased flow across the LVOT in utero. In addition, aortic stenosis leads to dilation and dysfunction of the left ventricle. Elevated end-diastolic pressures in the LV change flow dynamics such that there is left-to-right flow across the foramen ovale, with less systemic venous return directed into the left heart. In HLHS, this leads to severe mitral stenosis/atresia and poor development of the left ventricle with the consequence of ventricular hypoplasia. However, in patients with significant MR, such as in MVDS, there is an increased volume load on the left heart. The developing fetus with MVDS has both critical impediment to outflow across the aorta and severe MR with volume load. Therefore, there is continued, often progressive, left ventricular dilation and significant dysfunction. Severe incompetence of the dysplastic MV and MR lead to progressive left atrial dilation and left atrial hypertension. Elevated left atrial pressure may alter the geometry of the atrial septum with impetus for apposition of septum primum against septum secundum as left atrial pressure markedly exceeds right atrial pressure. This leads to progressive restriction, or at times complete closure, of the foramen ovale.
There are no identified extracardiac associations seen with MVDS, and no known genetic cause has been identified.
Prenatal Physiology
MVDS has a significant and profound impact on fetal cardiovascular physiology. As described previously, aortic stenosis results in little antegrade flow in the ascending aorta and the coronary arteries are primarily supplied via retrograde flow from the ductus arteriosus and right side of the heart. Aortic stenosis leads to left ventricular hypertrophy, dilation, and dysfunction. EFE limits diastolic relaxation of the ventricle. In addition, significant MR causes left atrial hypertension and severe left atrial dilation, sometimes to more than twice the size of the right atrium. Left heart dilation, left ventricular dysfunction, and significant MR are the key echocardiographic features of this disease in utero.
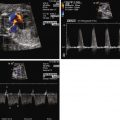
Key physiological features of MVDS include significant MR, left-to-right flow across the atrial septum, and atrial septal restriction. This restriction likely results from elevated left atrial pressures causing adherence of septum primum to septum secundum. With significant atrial septal restriction, elevated left ventricular filling pressures and significant MR contributing to left atrial hypertension, the pulmonary veins are engorged with limited ability to empty into the left atrium. Pulse wave Doppler sampling of the pulmonary veins often shows flow reversal during atrial systole. In addition, depending on the degree of atrial septal restriction and degree of MR, some patients can have blunting and/or reversal of pulmonary venous flow during ventricular systole. Hence, these fetuses have reversal of pulmonary venous flow with ventricular systole and with atrial contraction, revealing a unique “double-reversal” Doppler flow. This additional wave of retrograde pulmonary venous flow is unique to MVDS ( Figure 23-2 ).

The effects of left-to-right flow across the atrial septum and obstruction to pulmonary venous egress have a significant impact on fetal cardiovascular and lung development. However, given the unique ability of the fetus to redistribute blood flow, it is possible to have significant left atrial hypertension with restricted egress from the left atrium and yet no overt signs of altered cardiac output—hydrops fetalis—so long as the right side of the heart is functioning normally. Left-to-right flow at the level of the foramen ovale means that essentially all of the fetal cardiac output is provided via the right ventricle, with right-to-left flow across the patent ductus arteriosus. In addition, only approximately 10% to 25% of fetal cardiac output traverses to the pulmonary vascular bed and alterations in pulmonary venous return are of little clinical significance from the perspective of cardiac output during fetal life. However, pulmonary venous hypertension and impaired egress of blood from the left atrium can alter pulmonary vascular development creating a pulmonary vasculopathy, which becomes clinically apparent as soon as the fetus must use its own lungs, resulting in profound hypoxemia at umbilical cord separation at birth.
In some cases of MVDS, significant hydrops fetalis can occur. In these fetuses, hydrops likely results from massive left-sided dilation impinging upon filling of the right side with compression of the right ventricle. The inability of the right ventricle to fill raises right atrial pressure, leading to venous hypertension and hydrops. Because no effective perfusion can be achieved from the left side owing to the structural anomalies inherent in the disorder, right-sided function with ejection into the ductus arteriosus is vital for fetal survival. Cardiac output in such a scenario is diminished and fetal demise may occur. Alternatively, significant MR can lead to overall volume overload on the left heart and, if the foramen is patent, onto the right side of the heart as well. Animal studies have shown that the resting fetal heart is at near-maximum ventricular output and any increase in preload can exceed the fetal heart’s ability to increase stroke volume, leading to heart failure.
Despite these physiological changes, Doppler patterns of the umbilical artery and vein, ductus venous, and middle cerebral artery remain unchanged from normal in the majority of patients with MVDS, supporting the observation that the fetus can effectively redirect flow from the left to the right side of the heart. If right ventricular compression is present, changes in the ductus venosus consistent with right-sided restriction can be seen, and if cardiac output is limited, middle cerebral artery resistance will drop relative to umbilical artery resistance. In the presence of significant left atrial dilation in utero, arrhythmias may be observed in patients with this disorder. Of 24 prenatally diagnosed patients in two series reported on this disease, 2 patients had fetal arrhythmia, 1 with reentrant supraventricular tachycardia and the other with permanent junctional reciprocating tachycardia.
Prenatal Management
Serial fetal echocardiograms are essential to prenatal management and counseling of families who are carrying fetuses with MVDS. Identified fetal echocardiographic characteristics have been described that can predict patients who may need an immediate postnatal intervention to open the atrial septum and those who are at highest risk for poor outcome. In our review, we found that a total left heart area (traced left atrium plus left ventricle) that was 1.5 times the size of the right heart area (traced right atrium plus right ventricle) on the final fetal echocardiogram before birth was 100% predictive of mortality in the neonatal period. In addition, the presence of a “double-reversal” pulmonary venous Doppler flow pattern was 100% predictive of the need for an immediate postnatal intervention to open the atrial septum and overall mortality.
The most appropriate prenatal management of fetuses with MVDS remains unclear. Left unaltered, the natural history carries significant risk for infant mortality; therefore, in utero intervention aimed at altering the disease makes sense and would be an ideal option for patients with MVDS. In our center’s experience, prenatal intervention was undertaken in 3 of 10 patients ( Table 23-1 ). Two patients had balloon aortic valvuloplasty in an attempt to relieve aortic outflow obstruction, promote forward flow, and recruit the left ventricle with the hopes of also reducing the degree of MR. This strategy was utilized based on existing data that in utero aortic valvuloplasty may be a successful strategy for some patients in halting the progression of aortic stenosis toward HLHS. In both of these patients, the procedures were technically successful but also resulted a significant increase in aortic valve regurgitation (AR) without any change in the severity of the MR. The AR, in combination with continued MR, was a significant volume load on the left heart resulting in continued left ventricular dilation and severe dysfunction. Both patients underwent heart transplantation shortly after birth. One patient in our series had balloon atrial septostomy as a fetus, in order to compress the left atrium, potentially improve upon pulmonary vascular development, and potentially improve filling of the right heart thereby increasing cardiac output. In our case, there was technical difficulty in opening the atrial septum, which led to a fetal demise during the procedure. Other investigators have argued that opening the aortic valve and promoting forward flow alone or in combination with opening of the aortic valve may be helpful; however, mortality for this undertaking is quite high. Fetal hydrops was treated using maternal digoxin in 1 patient in our series, with some beneficial effect.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree