and Zdeněk Fryšák1
(1)
Department of Internal Medicine III – Nephrology, Rheumatology and Endocrinology, Faculty of Medicine and Dentistry, Palacky University Olomouc and University Hospital Olomouc, Olomouc, Czech Republic
Keywords
AmiodaroneThyrotoxicosisColor flow Doppler ultrasound6.1 Essential Facts
Amiodarone-induced thyrotoxicosis (AIT) develops in 3% of amiodarone-treated patients in North America. For those living in iodine-depleted areas, the incidence is higher (10%) and the risk also increases with increased dosage.
Amiodarone is a widely used class III antiarrhythmic drug used in the treatment of recurrent severe ventricular arrhythmias, paroxysmal atrial tachycardia, atrial fibrillation, and maintenance of sinus rhythm after atrial fibrillation cardioversion.
Thyrotoxicosis is mediated by amiodarone’s iodine content. Each 200 mg tablet contains 75 mg of iodine, and approximately 10% of this iodine is released as free iodide daily. Amiodarone accumulates in adipose tissue, cardiac and skeletal muscle, and the thyroid. With long-term treatment, there is a 40-fold increase in plasma and urinary iodide levels, and of elimination half-life of 50 to 100 days [1].
Amiodarone-induced thyrotoxicosis (AIT) is classified as type 1 or type 2 [1, 2]:
AIT type 1 occurs in patients with underlying thyroid pathology such as autonomous nodular goiter or Graves’ disease (GD) or Hashimoto’s thyroiditis (HT), in which iodine potentiates thyroid hormone synthesis and release.
AIT type 2 is a result of amiodarone causing a subacute destructive thyroiditis with release of preformed thyroid hormones into the circulation.
Occurrence of AIT is usually unpredictable, often sudden, and explosive, occurring either early or long after initiation of amiodarone treatment, mostly after 3 years of treatment. The median time of onset AIT type 1 is 3.5 months, and of AIT type 2, 30 months. It may also develop months after drug withdrawal [2].
The prevalence of the two main forms has changed over the last 30 years with a current predominance of the AIT type 2 [2].
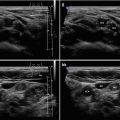
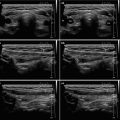
Diagnosis of AIT is based on clinical findings (signs and symptoms of thyrotoxicosis) and laboratory results, thyroid radioiodine uptake (RAIU) and US scan [2–4]:
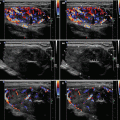
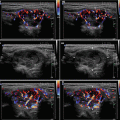
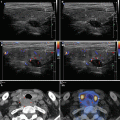
Diagnostic criteria AIT type 1 (Fig. 6.1aa): patients with GD, HT, solitary (≥ 1 cm), or multinodular goiter with corresponding laboratory and US findings, including an enhancement of vascularity at CFDS. AIT type 1 responds to combined thionamides and potassium perchlorate therapy.
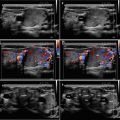
Diagnostic criteria AIT type 2 (Fig. 6.2aa): US criteria—normal or slightly increased thyroid volume without nodules (≥1 cm), at CFDS absent hypervascularity. Laboratory criteria—absence of circulating thyroid-directed autoantibodies [anti-thyroglobulin (Tg-Ab), anti-thyroid peroxidase (TPO-Ab), anti-TSH receptor (TSHR-Ab) and RAIU—low/undetectable thyroid radioiodine uptake (< 5% at 24 h). AIT type 2 is responsive to glucocorticoids.
Mixed form: A small subset of AIT type 1 patients may have a concomitant destructive process accountable for a longer onset time. This form requires a combination of the two therapeutic regimens.