Ankle and Foot
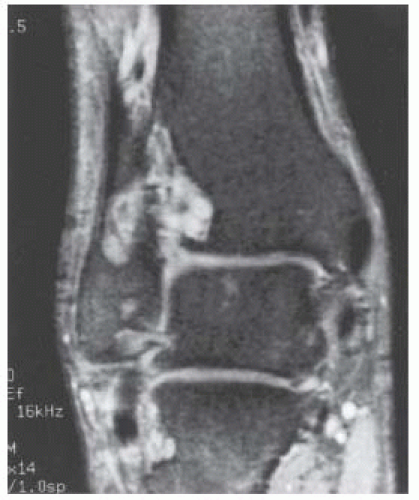
Case 8.1 PTT Longitudinal Split Tear
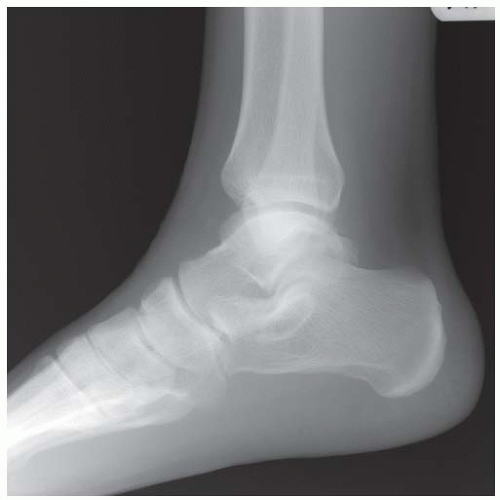
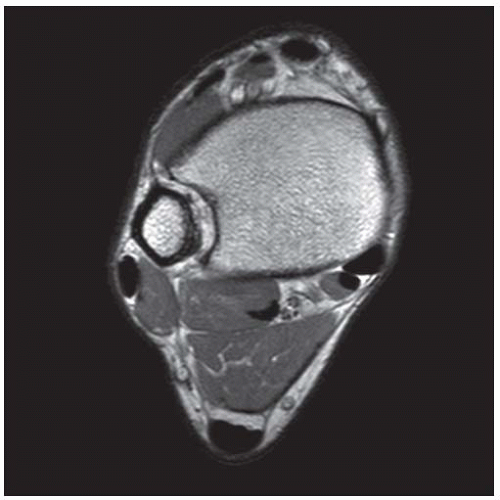
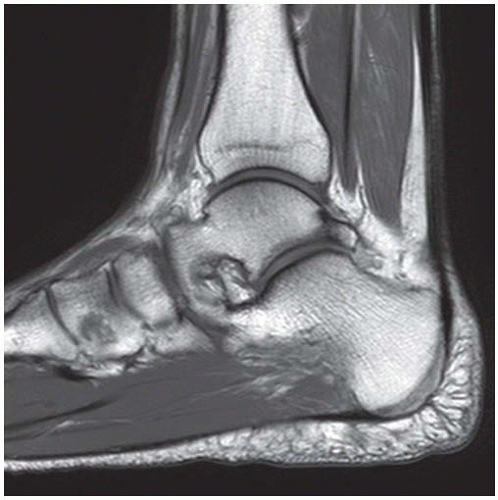
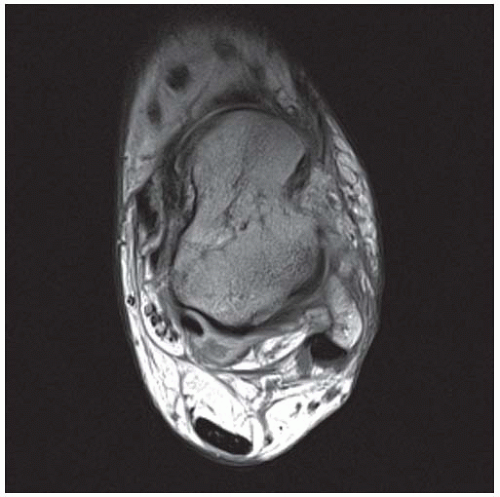
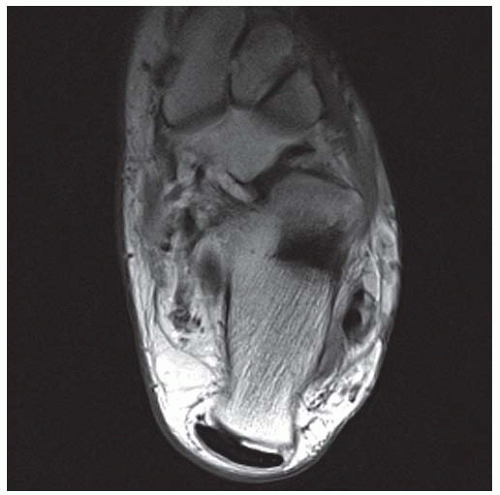
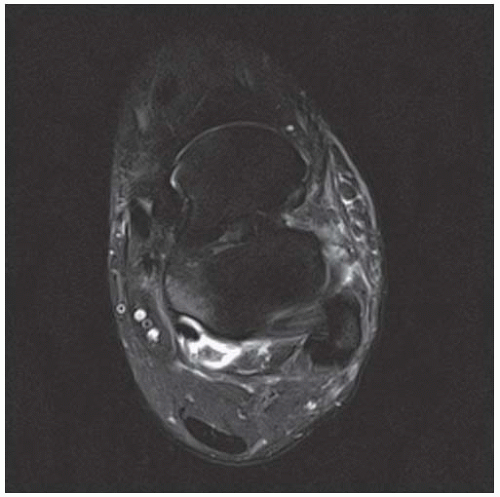
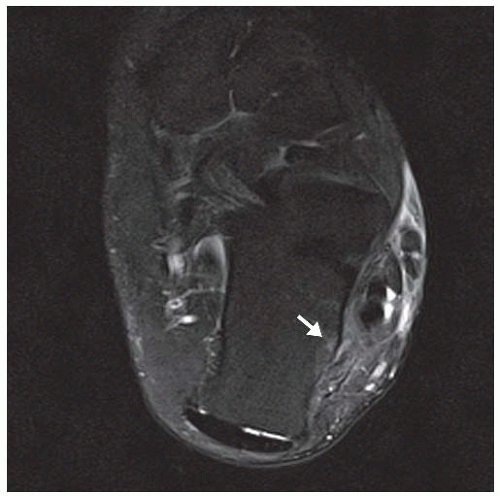
CLINICAL HISTORY A 71-year-old woman with ankle pain.
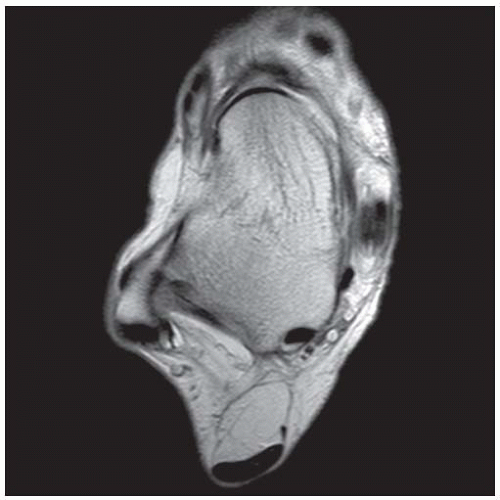
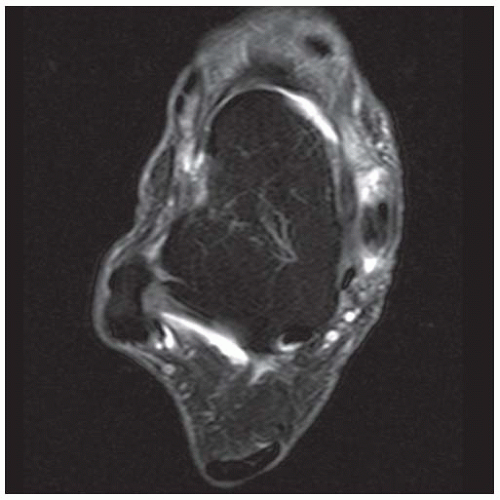
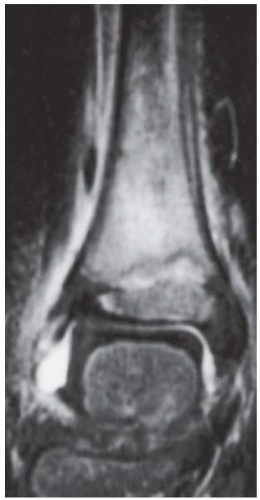
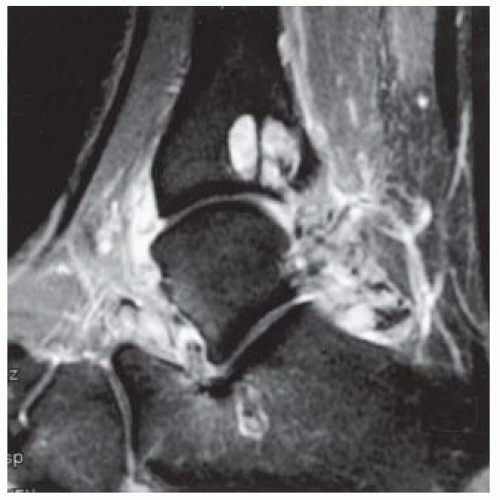
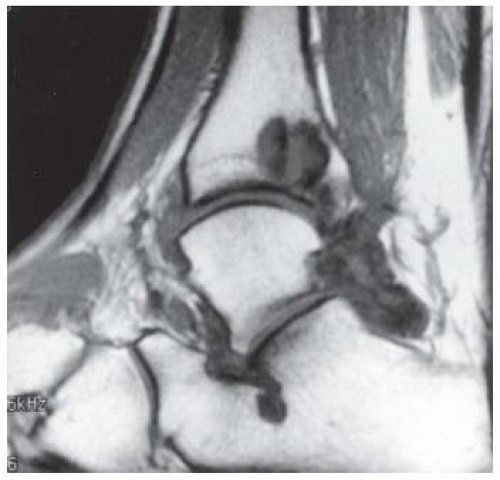
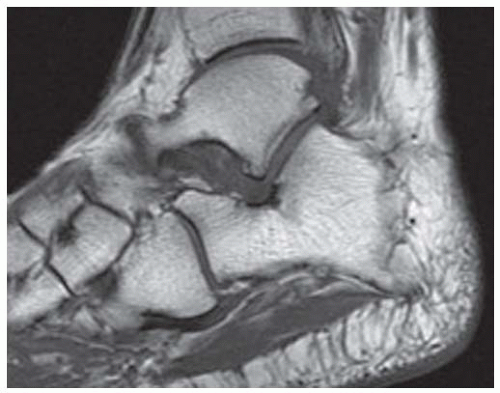
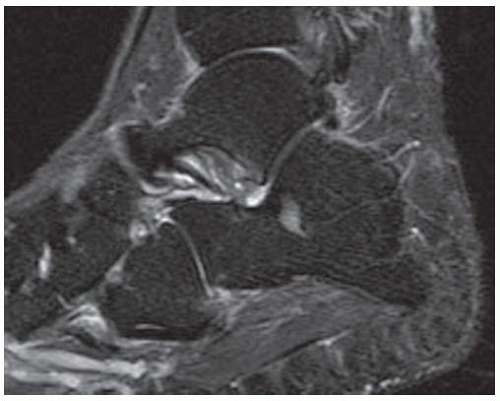
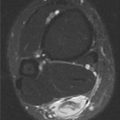
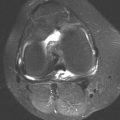
FINDINGS (A) Axial proton density and (B) T2-weighted, fat-suppressed magnetic resonance images (MRI) of the ankle show a thickened and split posterior tibial tendon (PTT). There is increased T2-weighted signal within the tendon with surrounding fluid in the sheath.
DIFFERENTIAL DIAGNOSIS None.
DIAGNOSIS PTT longitudinal split tear.
DISCUSSION The PTT is the largest and thickest of the three medial flexor tendons. The tendon courses under the medial malleolus and attaches to the medial navicular bone, the three cuneiforms, and the bases of the first to fourth metatarsals. Most PTT tears occur at the level of the medial malleolus. This tendon is one of the primary arch supporters for the foot. Tears of this tendon can cause flatfoot deformity and significant chronic pain and osteoarthritis [1]. Sinus tarsi syndrome has been described in association with PTT tear [2].
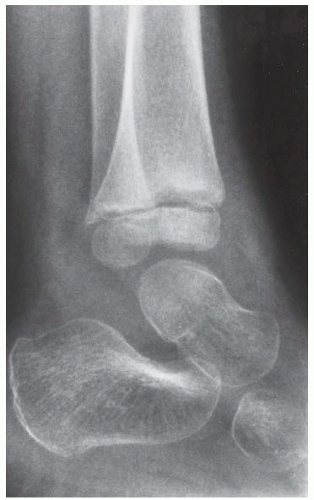
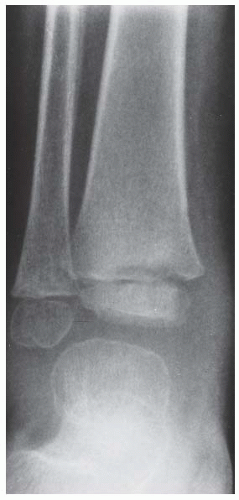
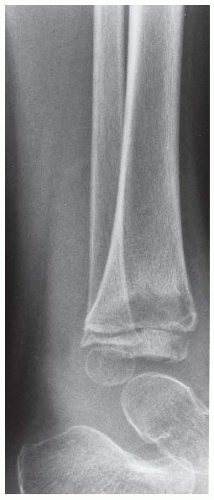
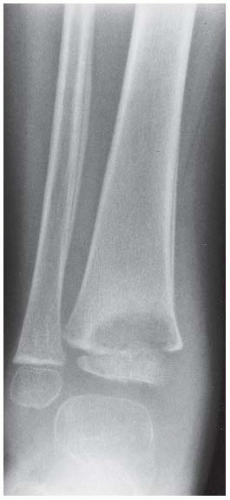
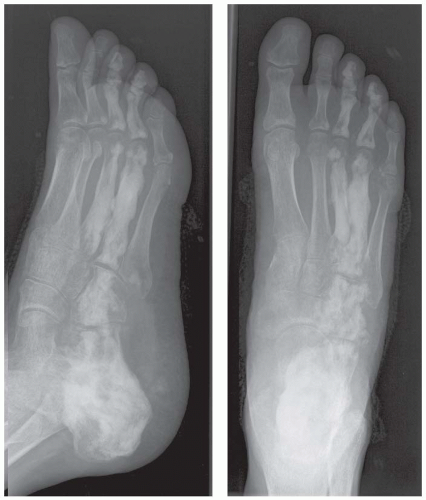
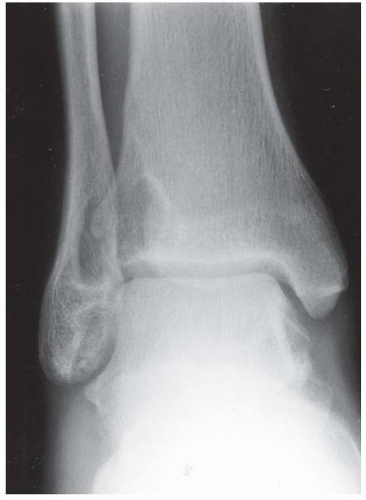
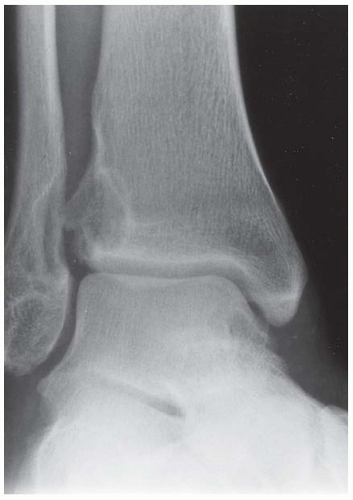
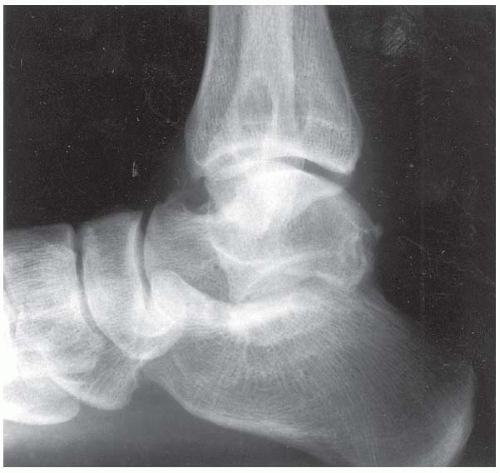
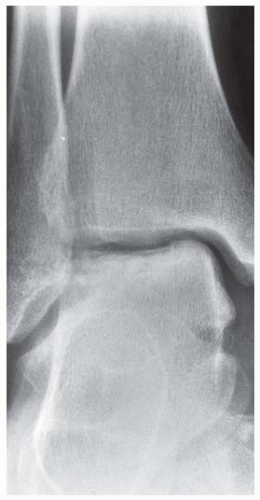
Case 8.2 Salter Type IV Fracture, Medial Malleolus
CLINICAL HISTORY A 9-year-old girl with ankle injury.
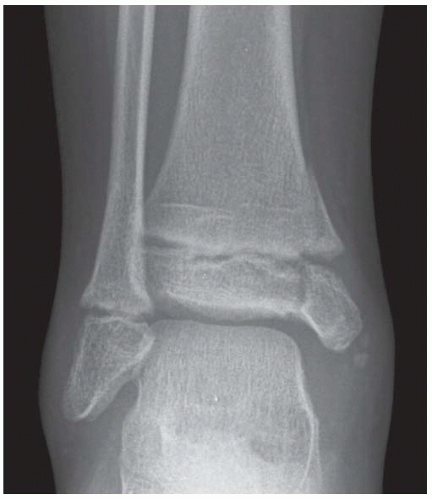
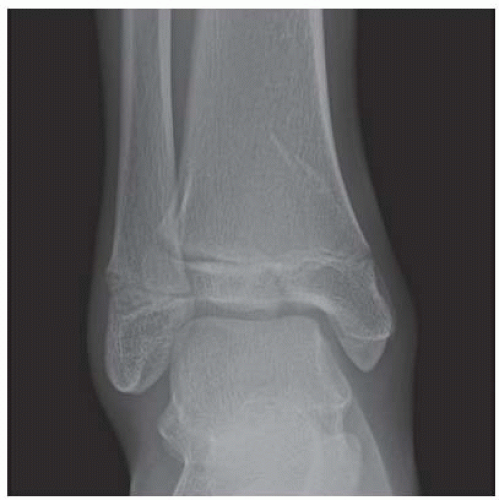
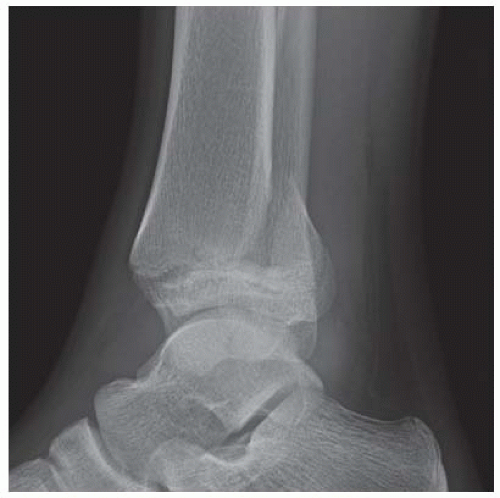
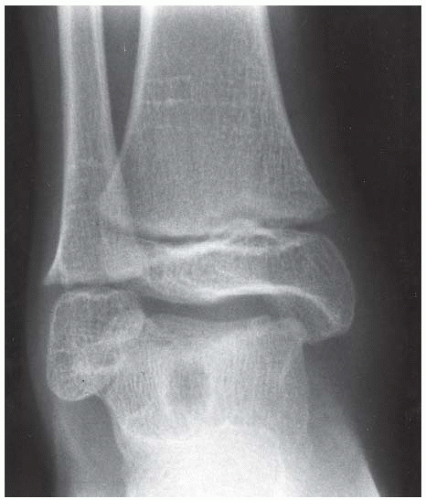
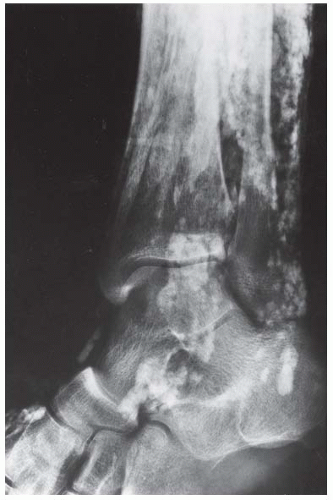
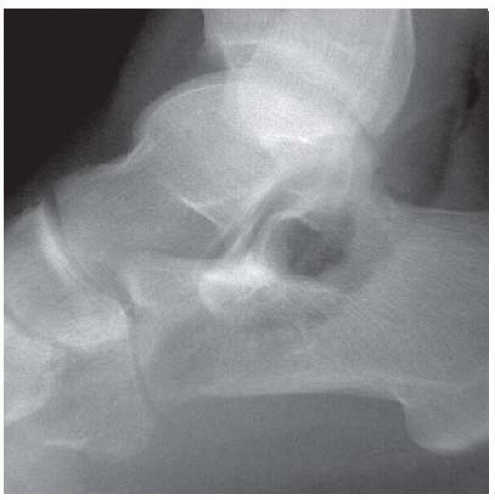
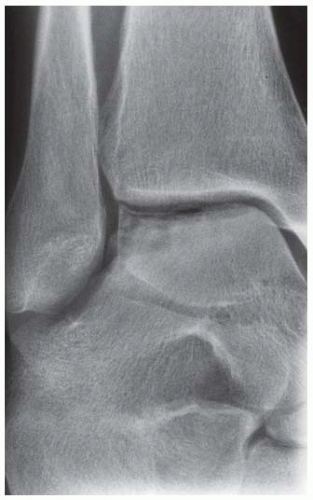
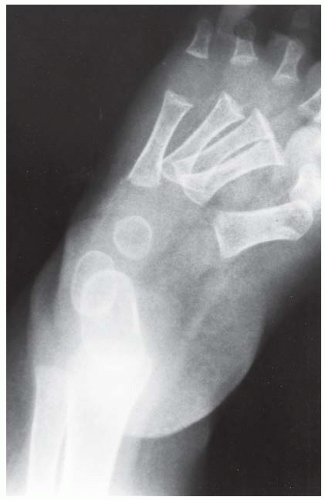
FINDINGS Anteroposterior (AP) ankle radiograph shows an intra-articular fracture plane extending vertically through the medial aspect of the tibia, passing through the epiphysis, growth plate, and metaphysis, and separating the medial malleolus from the remainder of the tibia.
DIFFERENTIAL DIAGNOSIS None.
DIAGNOSIS Salter type IV fracture, medial malleolus.
DISCUSSION Salter type IV fractures pass through the metaphysis, growth plate, and epiphysis. In this case, a vertical fracture in the sagittal plane can be seen passing through these three structures. These fractures require accurate open reduction and internal fixation (ORIF), because misalignment of the principal fragments would allow a bone bridge to form across the growth plate [3]. Such a bone bridge would tether the growth plate on one side and result in a progressive varus deformity as the open, lateral portion of the growth plate continued to lengthen the bone. The potential for deformity is greater in young children, who have a longer remaining growth period. The risk of complications is high if the fracture has more than 2 mm of displacement. A Salter type I or type II fracture of the fibula may be associated with this injury.
Case 8.3 Triplane Fracture
CLINICAL HISTORY A 14-year-old boy with lacrosse injury.
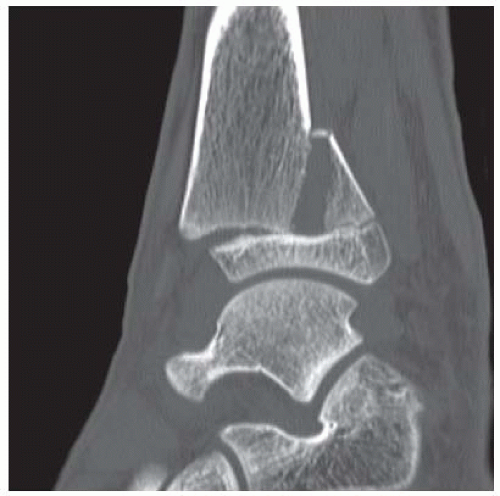
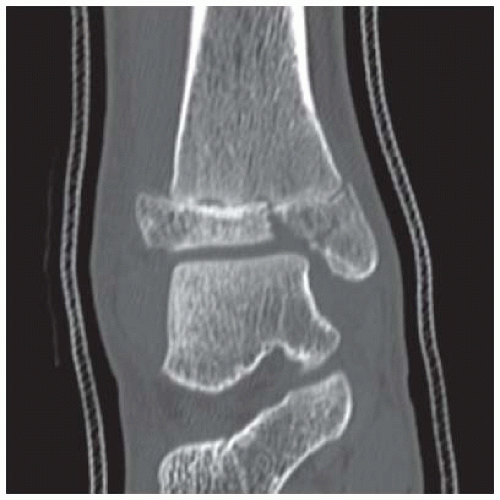
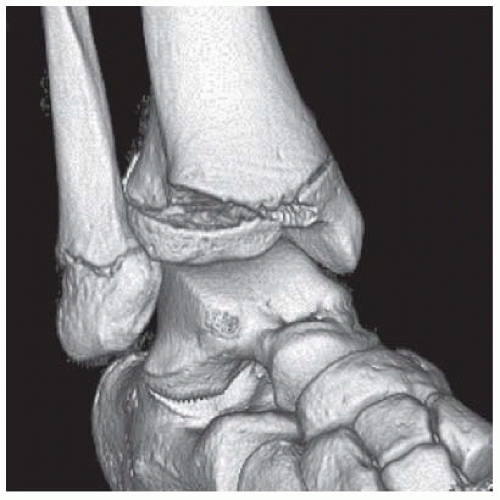
FINDINGS (A, B) AP, lateral radiographs, (C) sagittal, (D) coronal reformatted, and (E) three-dimensional volume rendering computed tomography (CT) of the right ankle demonstrate a vertical fracture through the epiphysis that runs from anterior to posterior, and an oblique fracture through the posterior distal tibial metaphysis. The medial malleolus is intact, as is the lateral metaphysis. The central part of the distal tibial growth plate is closed, the most medial portion of the growth plate is open, and the lateral portion is abnormally widened.
DIFFERENTIAL DIAGNOSIS Salter type II fracture, Salter type III fracture, Salter type IV fracture, juvenile Tillaux fracture, triplane fracture.
DIAGNOSIS Triplane fracture.
DISCUSSION The radiographs and CT show fractures of the distal tibia that appear to involve the growth plate. One fracture component extends through the metaphysis in the oblique coronal plane, and one fracture extends through the epiphysis in the sagittal plane. Because the fracture through the growth plate that connects the metaphyseal and epiphyseal fractures is in the axial plane, the components of this fracture occur in three different planes, defining the diagnosis of a triplane fracture. The triplane fracture has elements of Salter type II and type III fractures, and because it crosses the epiphysis, growth plate, and metaphysis, it can also be considered a Salter type IV fracture. The juvenile Tillaux fracture, in comparison, is a fracture in which the anterolateral aspect of the tibia epiphysis is avulsed by tension from the anterior tibiofibular ligaments during external rotation of the foot; the juvenile Tillaux fracture can be classified as a Salter type III fracture.
Triplane fractures only occur when physeal closure of the distal tibia is incomplete [4,5]. The distal tibial growth plate begins to close centrally, then medially, and finally laterally. Plantar flexion and external rotation are believed to be the mechanisms of injury in the triplane fracture, in which the lateral fragment of tibia that includes a portion of the epiphysis and metaphysis is avulsed by tension on the tibiofibular ligaments. Fracture of the fibula may accompany these injuries. When the separated epiphysis has two fragments, one of which has a portion of posterior tibial metaphysis attached to it and the other comprises a fragment of the medial epiphysis, a three-part triplane fracture is produced. Three-part fractures are usually more severe than two-part fractures, since there is greater displacement of the epiphyseal fragments. ORIF is used more frequently for three-part injuries.
The mean age at the time of fracture is about 15 years in boys and 13 years in girls, with a range from about 12 to 17 years in boys and 10 to 15 years in girls. Triplane fractures comprise 15% and 7% of ankle fractures in boys and girls under the age of 18 years, respectively, but the proportion would be much higher in the age range in which these fractures occur. If an epiphyseal gap of 3 mm or greater remains after ORIF, there is a greater risk of premature physeal closure [6].
Case 8.4 Atypical Mycobacterium Osteomyelitis
CLINICAL HISTORY A 4-year-old boy who refuses to walk. A-B, 2 weeks after presentation; C-F, 6 weeks after presentation.
FINDINGS
A, B. Lateral and AP radiographs taken 2 weeks after initial presentation demonstrate a faint oval lucency of the distal tibial metaphyses with surrounding sclerosis. Soft tissue swelling is noted. Initial radiographs taken 2 weeks earlier were normal (not shown).
C, D. Radiographs taken 6 weeks after presentation show that the lucency has grown larger and more defined. Periosteal reaction extends up the shafts of the tibia and fibula. The soft tissue swelling is marked.
E, F. T2-weighted MRI taken 6 weeks after presentation demonstrates a focal area of increased signal in the distal tibial metaphysis, adjacent to but not crossing the growth plate, corresponding to the lucency noted on plain film. An ankle effusion and soft tissue edema is evident.
DIFFERENTIAL DIAGNOSIS Growth plate fracture; osteomyelitis caused by pyogenic, granulomatous, or fungal organism; malignant tumor such as osteosarcoma, lymphoma, or Ewing sarcoma.
DIAGNOSIS Atypical mycobacterium osteomyelitis.
DISCUSSION A growth plate fracture of the tibia in an otherwise healthy child should be healed at 6 weeks, although at 2-week follow-up, Salter type I fracture might show widening of the growth plate and sclerosis on the metaphyseal side. The asymmetry of the widening and sclerosis would be unusual for trauma. The enlarging lucency in the metaphysis suggests a destructive process such as osteomyelitis or tumor. The location at the distal metaphysis is appropriate for hematogenous osteomyelitis, but the modesty of the reactive bone and the lack of involvement of the physis and epiphysis at the end of 6 weeks would be uncharacteristic of pyogenic osteomyelitis. The small size of the lesion would be distinctly unusual for a primary malignant bone tumor such as osteosarcoma, lymphoma, or Ewing sarcoma, although the metaphyseal sclerosis could represent neoplastic bone. The diagnosis in this case was confirmed at open biopsy. The lucency consisted of a fluid-filled cystic space, and cultures of the fluid were positive.
Nontuberculous mycobacterial musculoskeletal infections may take the form of tenosynovitis, synovitis, or osteomyelitis [7]. The spectrum of pathologic findings includes virtually no inflammation, mild-to-severe nonspecific chronic infection, granulomas without necrosis, and caseating epithelioid granulomas indistinguishable from tuberculosis. These pathologic changes explain the blandness of the imaging features in this case. Multiple lesions in the metaphyses and diaphyses, sinus tracts, and osteoporosis are other features of atypical mycobacterial infections. Joint space narrowing, as in tuberculosis, is a late finding. Delays in the diagnosis of atypical mycobacterial osteomyelitis are common, and the disease may be multifocal. These infections occur in both immunocompromised as well as immunocompetent individuals [8].
Case 8.5 Extraskeletal Primitive Neuroectodermal Tumor (PNET)
CLINICAL HISTORY A 29-year-old man with lower leg swelling.
FINDINGS
A. Sagittal T1-weighted MRI shows a mass in the distal soleus muscle, extending inferiorly along the deep aspect of the Achilles tendon and abutting the posterior aspect of the tibia and ankle. The mass has a lobular contour and heterogeneous intermediate signal intensity.
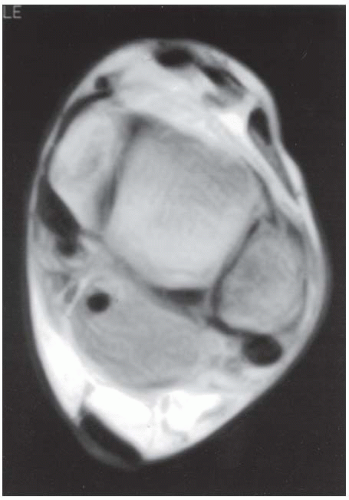
B. Axial T1-weighted MRI at the level of the ankle joint shows the lesion in the posterior soft tissues, adjacent to the joint and surrounding the tendon of the flexor hallucis longus. The underlying bone is normal.
DIFFERENTIAL DIAGNOSIS Soft tissue sarcoma, extraskeletal Ewing sarcoma, lymphoma, benign soft tissue tumor, myotendinous injury, myositis ossificans, hematoma.
DIAGNOSIS Extraskeletal primitive neuroectodermal tumor (PNET).
DISCUSSION The finding of a large, lobulated, soft tissue mass in the deep tissues of the extremity of an adult is always cause for concern. There are no specific features on imaging that lead to the diagnosis in this case, but myotendinous injury, hematoma, and myositis ossificans are possibilities that should have a suggestive history (lacking in this case). The age of the patient is less than the peak incidence for a mesenchymal soft tissue sarcoma. A biopsy is necessary for diagnosis.
PNET is a sarcoma of neuroectodermal origin. It appears closely related to Ewing sarcoma in histogenesis [9], with an appearance on light microscopy that may be indistinguishable (small, round, blue cells). Although both Ewing sarcoma and PNET may be primary to bone or soft tissue, Ewing sarcoma is much more likely to occur in bone, whereas PNET has an equal incidence in bone and soft tissue. Most patients with PNET are between 10 and 30 years old, with the most common soft tissue sites being the paravertebral region, the chest wall (called in this circumstance an Askin tumor), the retroperitoneum, and the lower extremities. Soft tissue PNETs present as rapidly enlarging soft tissue masses. Findings on imaging are nonspecific, and PNET is indistinguishable from Ewing sarcoma on imaging [10]. The margins of the tumor may be well defined, as in this case, and sometimes encapsulated, or they may appear infiltrative. Areas of hemorrhage may be present, and contrast enhancement on MRI is typically intense. The treatment of PNET is chemotherapy and surgical resection, sometimes with radiation therapy as well. The prognosis for patients with PNET is less favorable than for patients with Ewing sarcoma [11].
Case 8.6 Accessory Soleus Muscle
CLINICAL HISTORY A 33-year-old man with ankle pain.
FINDINGS
A. Lateral radiograph of the ankle demonstrates soft tissue mass partially occupying the Kager’s fat pad, posterior to the flexor hallucis longus.
B, C. Axial and sagittal T1-weighted MRI shows accessory muscle anterior to the Achilles tendon.
DIFFERENTIAL DIAGNOSIS None.
DIAGNOSIS Accessory soleus muscle.
DISCUSSION According to cadaveric studies, an accessory soleus has a prevalence of 0.7% to 5.5%, with the muscle most commonly seen as a unilateral finding [12]. It arises from the deep surface of the soleus or from the fibula and the tibia. From its origin, the muscle descends anterior or anteromedial to the Achilles tendon. The accessory soleus is generally enveloped within its own fascia and derives its blood supply from the posterior tibial artery. The posterior tibial nerve supplies both the soleus proper and the accessory soleus muscle [13].
An accessory soleus may manifest clinically as a soft tissue mass in the ankle. Clinically evident accessory soleus muscles have a male predilection and commonly manifest in the 2nd and 3rd decades of life. On cross-sectional imaging the accessory muscle is demonstrated anterior to the Achilles tendon and superficial to the flexor retinaculum, typically extending medially to the area between the medial edge of the Achilles tendon and the medial malleolus. Treatment usually depends on the presence or severity of the symptoms. For symptomatic patients, conservative treatment or surgical approaches including fasciotomy or excision of the muscle can be performed [14].
Case 8.7 Lateral Ankle Ligament Tear
CLINICAL HISTORY A 28-year-old woman with ankle sprain.
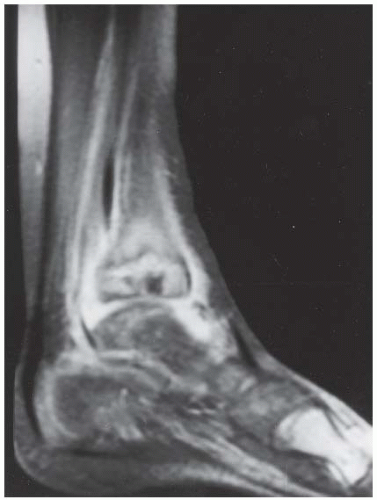
FINDINGS (A, B) Axial proton density and (C, D) T2-weighted fat-suppressed MRI demonstrates complete tear of anterior talofibular (ATF) ligament surrounded by fluid signal. The calcaneofibular (CF) ligament is also torn underneath the peroneus tendon sheath. The posterior talofibular (PTF) ligament shows increased signal intensity with preserved fibers consistent with sprain. Also, noted is bone marrow edema in the posterior talus and peroneal tubercle of calcaneus.
DIFFERENTIAL DIAGNOSIS None.
DIAGNOSIS Lateral ankle ligament tear.
DISCUSSION The lateral collateral ligament complex is made up of three ligaments: the ATF, CF, and PTF ligaments. Injuries to this complex are very common, especially in the young, active population. Of these ligaments, the ATF ligament is the weakest and most easily torn. As inversion stress increases, the CF ligament becomes torn. PTF ligament injury is uncommon, and is almost always associated with injury to both the ATF and CF ligaments. Acute grade I and II tears, stretching and partial tears, are often treated conservatively. The treatment of grade III tears, complete rupture of a ligament, is more controversial. Regardless of the treatment, the prognosis of most ankle sprains is quite good. Unfortunately, 10% to 20% of patients develop chronic instability [15]. MR arthrography of the ankle has been shown to be a highly sensitive modality for detecting and accurately staging lateral ankle ligament tears. MR arthrography allows the evaluation of the torn free ends of the ligaments to assess the feasibility of primary repair and the evaluation of adjacent structures, which may potentially be used in surgical reconstruction [16].
Case 8.8 Melorheostosis
CLINICAL HISTORY A 34-year-old woman with foot pain.
FINDINGS
A. Oblique and AP radiographs of the foot demonstrate wavy, dense sclerosis beginning in the calcaneus, involving the cuboid, and extending through the third and fourth metatarsals and toes.
B. Long-axis CT shows the sclerosis involving the cortex and medullary canal. There is no soft tissue mass or bone destruction.
DIFFERENTIAL DIAGNOSIS Melorheostosis, trauma, chronic osteomyelitis, osteosarcomatosis.
DIAGNOSIS Melorheostosis.
DISCUSSION Melorheostosis is a disorder of unknown cause that can present at any age. The most common appearance is cortical hyperostosis that can be likened to dripping candle wax. Involvement is usually limited to a single extremity and is often along a sclerotomal distribution [17], in this case L5. When the soft tissues are involved, muscle and tendon contractures can lead to skeletal deformities such as scoliosis and growth disturbances. Most patients are asymptomatic, but this condition can painful [18]. Diseases associated with melorheostosis include osteopoikilosis, osteopathia striata, neurofibromatosis, and tuberous sclerosis. Treatment is most often surgical, to correct deformities and remove soft tissue masses [19].
Case 8.9 Hemophiliac Pseudotumor
CLINICAL HISTORY An 8-year-old boy with ankle pain.
FINDINGS
A. AP radiograph of the ankle shows epiphyseal overgrowth, flattening of the talar dome, sclerosis, and cystic changes. A 1-cm lucency with sclerotic borders is noted in the medullary space of the talus.
B. CT of both ankles demonstrates a well-defined ovoid lucent lesion in the right talus with surrounding sclerosis. The lesion has soft tissue attenuation within. Articular irregularity is present at the dome of the talus, and the talus appears mildly contracted in size compared with the contralateral asymptomatic side. Soft tissue density within the ankle joint may represent fluid or synovial hypertrophy.
DIFFERENTIAL DIAGNOSIS Hemophilia, juvenile idiopathic arthritis, septic arthritis, sickle cell disease.
DIAGNOSIS Hemophiliac pseudotumor.
DISCUSSION Abnormality of the articular surface of bone associated with synovial hypertrophy in a pediatric patient can be seen in juvenile idiopathic arthritis, hemophilia, and the rheumatoid-negative spondyloarthropathies. The cystic lesion is remote from the subarticular bone, which would be unusual for a subchondral cyst in the arthritic conditions mentioned, but could represent a pseudotumor from intraosseous hemorrhage in hemophilia.
Intraosseous bleeding and the subsequent inflammatory reaction that clears the hemorrhage may create radiolucent defects in bone, called pseudotumors. Repeated bleeding into a pseudotumor may cause it to recur and enlarge, simulating malignancy. Osteonecrosis or osteomyelitis in the setting of sickle cell disease involving the talus may have a similar appearance to this case. Septic arthritis may also result in osteomyelitis and osteonecrosis. Radiographic manifestations in hemophilia may be strikingly similar to certain arthritic conditions, particularly juvenile idiopathic arthritis. Because the pathomechanical basis for these findings differs (intra-articular hemorrhage with secondary inflammation versus chronic inflammation without hemorrhage), features specific for hemorrhage should be sought when attempting to distinguish between these diagnoses. The obvious differential point is the presence of a hemorrhagic effusion, which can be ascertained on MRI, but other differentiating features include localized periosteal reaction due to subperiosteal hemorrhage and pseudotumor due to intraosseous hemorrhage.
Soft tissue hemorrhage may result in a soft tissue pseudotumor, which can cause pressure erosions and scalloping on the adjacent bone with or without exuberant periosteal reaction, again simulating neoplasm. Most pseudotumors arise near the large bones of the proximal skeleton in adults, but in younger patients, before skeletal maturity, they tend to occur distal to the wrist or ankle. Distal pseudotumors usually respond well to conservative management, whereas resection is often the treatment of choice for proximal pseudotumors [20]. Large proximal pseudotumors may lead to life-threatening hemorrhage. Emergent surgical treatment carries a significant intraoperative mortality.
Case 8.10 Osteonecrosis in Sickle Cell Disease
CLINICAL HISTORY A 10-year-old boy with ankle pain.
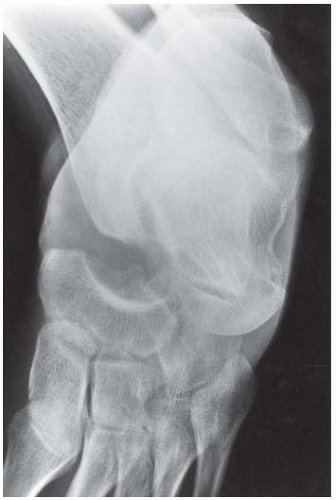
FINDINGS (A, B) Lateral and mortise radiographs of the ankle show disordered mineralization in the distal tibial epiphysis. The epiphysis is ossifying from multiple centers. There is a dense horizontal line of sclerosis across the distal tibial metaphysis. The ossification patterns in the medial and lateral malleoli are also disordered.
DIFFERENTIAL DIAGNOSIS Osteonecrosis, epiphyseal dysplasia, trauma, osteomyelitis.
DIAGNOSIS Osteonecrosis in sickle cell disease.
DISCUSSION The appearance of the epiphysis with multiple centers of ossification is that of revascularized osteonecrosis. In this case, the patient was known to have sickle cell disease, and the clinical question was the cause of his ankle pain. Sickle cell disease is caused by an inherited structural defect in hemoglobin that leads to red cell dysfunction. The radiologic features of sickle cell disease in bone are the result of bone marrow hyperplasia, vascular occlusion, and osteomyelitis [21]. Hyperplasia of the bone marrow expands the marrow space. Vascular occlusion results in osteonecrosis. Any portion of any bone may be infarcted; frequent sites include the medullary space of long bones, growing epiphyses, and the hands. Involvement of growing epiphyses leads to growth disturbances. If the femoral head is involved, the pathophysiologic events and sequelae are indistinguishable from those of Legg-Calvé-Perthes disease. A growth disturbance in the vertebral body leads to development of H-shaped vertebrae. Localized infarctions of bone with repair or dystrophic calcification result in focal areas of bony sclerosis scattered about the skeleton.
Patients with sickle cell disease have a high incidence of osteomyelitis. Unlike hematogenous osteomyelitis in other situations, the infection in sickle cell disease is most frequent at the diaphysis of the long bones, where the oxygen tension is lowest. In approximately 50% of cases, Salmonella species or mixed flora are the causative organisms (their presence is exceedingly unusual under any other circumstance); the remaining cases are usually caused by Staphylococcus species. Chronicity and recurrence are common. Osteomyelitis may be difficult to differentiate from infarction on both clinical and radiologic grounds [22,23]; either one can be a complication of the other. The radiographic signs of osteomyelitis would superimpose on whatever preexisting bone changes existed from the sickle cell disease.
Case 8.11 Marrow Infarction
CLINICAL HISTORY A 63-year-old man with newly diagnosed prostate cancer.
FINDINGS
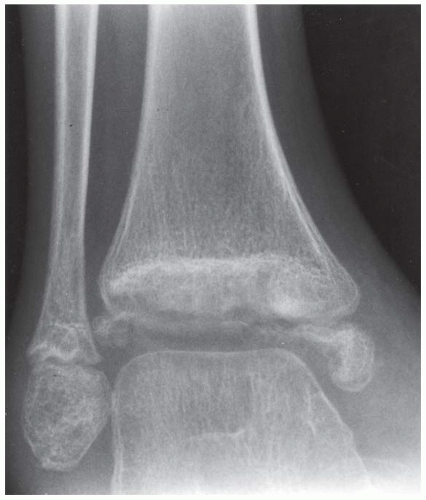
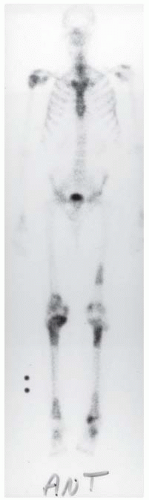
A. Whole-body radionuclide bone scan demonstrates increased uptake in several locations, with particular involvement of the ends of the bones (bilaterally in the tibias, distal femurs, right proximal humerus, tali, and left distal femoral shaft). The sites of involvement are relatively large. The axial skeleton appears spared.
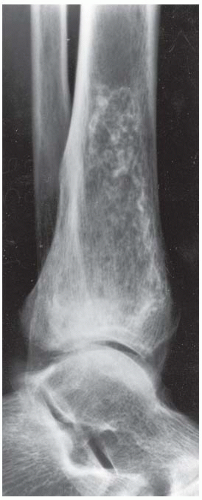
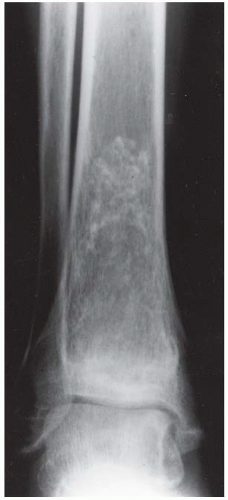
B, C. Two radiographic views of the distal right tibia demonstrate a lesion with a lacy calcified matrix and a sclerotic serpiginous rind. There is no cortical expansion or penetration.
DIFFERENTIAL DIAGNOSIS Metastases, enchondroma, marrow infarction (osteonecrosis).
DIAGNOSIS Marrow infarction.
DISCUSSION Metastases typically involve the axial skeleton more than the appendicular skeleton. Lesions in the ankle and feet are unusual. Metastases also tend to be focal and discrete. Enchondroma and marrow infarcts can demonstrate radionuclide accumulation on bone scans. Accumulation in enchondromas is a reflection of reactive bone formation and enchondral ossification. Accumulation in infarcts is a reflection of repair and remodeling after revascularization. During the ischemic phase of marrow infarction, the bone scan may be cold or normal. Enchondroma and marrow infarction may have a similar radiographic appearance. The serpiginous rind of calcification and the location at the end of the bone is more suggestive of marrow infarction than enchondroma, and the multifocal involvement is more common in marrow infarction than in enchondroma.
There are multiple potential causes for marrow infarction, including exogenous steroids, alcoholism, sickle cell disease, trauma, Gaucher disease, and pancreatitis. Abnormal fat metabolism in alcoholism and pancreatitis is believed to cause osteonecrosis by fat embolism. On MRI, medullary infarcts are usually multiple and have a diagnostic appearance. They have an irregular, serpiginous, sharply defined, low-signal border on T1-weighted and proton-density images, which often has high signal on T2-weighted images. This appearance corresponds to the margin of revascularization and remodeling. Contemporaneous radiographs are often normal, but the infarcted marrow may eventually calcify. This dystrophic calcification in infarcts may resemble the mineralized matrix of an endosteal cartilage tumor.
Case 8.12 Pigmented Villonodular Synovitis (PVNS)
CLINICAL HISTORY A 32-year-old woman with chronic swelling and ankle pain.
FINDINGS
A, B. AP and mortise radiographs of the ankle demonstrate erosive changes in the tibia and fibula. The erosions are shallow and have sclerotic margins. The cartilage space is preserved. The distribution of abnormality suggests a primary synovial process rather than a multifocal bone process.
C. Lateral radiograph shows erosive changes of the talus along the anterior superior aspect as well as the posterior aspect, indicating a diffuse process.
D-F. Coronal fat-saturated T2-weighted MRI, sagittal fat-saturated T2-weighted MRI, and sagittal T1-weighted MRI demonstrates profuse synovial hypertrophy and associated lesions in the subcortical bone, some of which appear to be communicating with the joint. There are low-signal-intensity foci within the hypertrophied synovium and within the intraosseous lesions.
DIFFERENTIAL DIAGNOSIS Pigmented villonodular synovitis (PVNS), synovial osteochondromatosis, amyloid arthropathy, tuberculosis, synovial hemangiomatosis.
DIAGNOSIS Pigmented villonodular synovitis (PVNS).
DISCUSSION All of the entities in the differential diagnosis can demonstrate synovial hypertrophy and bony erosions. The lack of calcifications on the radiographs makes synovial osteochondromatosis much less likely. The low-MR-signal-intensity foci within the synovium and bone on T2-weighted images may be seen with PVNS or amyloidosis, but the monoarticular nature of the process favors PVNS over amyloidosis. These low-signal foci would not be expected with tuberculosis or hemangiomatosis.
PVNS is believed to be a benign neoplasm of the synovium. It is typically a monoarticular process that has a predilection for the large joints of the lower extremity, especially the knee [24]; however, any synovial joint may be involved. Radiographic findings include joint effusion, juxta-articular erosions, and relative sparing of the cartilage space. In later stages, secondary osteoarthritis may be seen. Ankylosis is not a feature. The synovial masses generally do not calcify or ossify in PVNS, an important point of differentiation from synovial osteochondromatosis [25]. On MRI, PVNS demonstrates foci of low signal intensity on all pulse sequences due to widespread hemosiderin deposits from repeated episodes of intra-articular hemorrhage. These hemosiderin deposits also give the synovium a pigmented appearance at gross inspection. Amyloidosis may show a similar pattern of low-signal foci on MRI, but amyloidosis is most often a systemic, multiarticular process that is related to long-term hemodialysis [26].
PVNS is treated by synovectomy, but because complete surgical removal is difficult, recurrence rates may be as high as 50%. Development of malignancy in the setting of PVNS is an extremely rare event. Cases of malignant PVNS have been reported in patients with previously documented and treated benign PVNS, as well as in patients with PVNS and malignancy at initial presentation [27,28].
Case 8.13 Dermatomyositis
CLINICAL HISTORY A 26-year-old woman with skin problems.
FINDINGS Oblique ankle radiograph. There is amorphous dense soft tissue calcification, distributed in vertical sheets. No underlying osseous abnormality is identified. No joint disease is present.
DIFFERENTIAL DIAGNOSIS Dermatomyositis, systemic lupus erythematosus (SLE), scleroderma, hypervitaminosis D, hyperparathyroidism, pseudohypoparathyroidism, pseudopseudohypoparathyroidism, parasitic infection.
DIAGNOSIS Dermatomyositis.
DISCUSSION Extensive soft tissue calcification is present, some of which is subcutaneous, but some appears to be intramuscular and possibly intratendinous. The calcifications are amorphous in character, without defined internal structure. Numerous disorders can be associated with soft tissue calcifications, including dermatomyositis, SLE, scleroderma, hypervitaminosis D, hyperparathyroidism, pseudohypoparathyroidism, pseudopseudohypoparathyroidism, and parasitic infection.
Dermatomyositis is an idiopathic disorder with non-infectious inflammation of the skin and skeletal muscle. Polymyositis involves muscle alone. Multiple types of the disease have been described based on the clinical presentation, and a clinical form associated with underlying malignancy has been described. Regardless of subtype, nearly all patients experience muscle weakness, usually proximal. Skin rashes and arthralgias are common clinical findings and may be the presenting symptoms. Involvement of cardiac, pulmonary, renal, neurologic, and gastrointestinal tissues will alter the presentation. Laboratory tests reveal elevation of creatine kinase during active inflammation, and electromyographic alterations can be detected. Soft tissue findings tend to be more common and severe in children than adults. Soft tissue swelling may be appreciated by dedicated soft tissue films in areas of complaint, but soft tissue calcifications represent the most dramatic radiographic finding. Distinct patterns of soft tissue calcification have been described, the most common of which are linear, sheet-like calcification in the interfascial planes. Reticular and rounded calcifications may be seen in the subcutaneous tissues, and deep rounded calcifications are common. In childhood disease, spontaneous remission of soft tissue calcifications has been reported with the onset of puberty. Joint changes on radiographs are rare and usually limited to soft tissue swelling. Distal tuft resorption may be seen in association with soft tissue calcification, which may be confused with scleroderma.
MRI is a useful method for evaluating the extent and activity of disease. Actively involved muscle tends to demonstrate increased signal on T2-weighted images; inversion recovery or fat-suppressed, T2-weighted images are the most sensitive method for displaying these changes [29]. Antimyosin scintigraphy may be even more sensitive than MRI for detecting active disease [30]. Identification of active disease is useful for directing biopsy.
Case 8.14 Sinus Tarsi Syndrome
CLINICAL HISTORY A 73-year-old woman with persistent lateral foot pain after twisting her ankle.
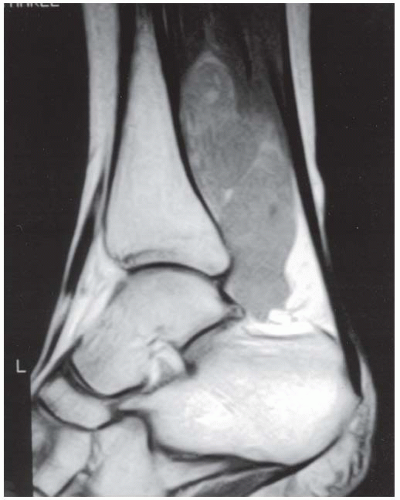
FINDINGS (A, B) Sagittal T1-weighted and inversion recovery MRI of the hindfoot shows edema and resultant loss of the normal fat signal within the sinus tarsi region.
DIFFERENTIAL DIAGNOSIS None.
DIAGNOSIS Sinus tarsi syndrome.
DISCUSSION The sinus tarsi is the space between the talar neck and distal calcaneus. This space contains ligaments, neurovascular structures, and fat. In a severe ankle inversion injury, inflammation or hemorrhage in this region may produce chronic pain. The ligaments within the sinus tarsi may also be torn, producing a sensation of instability. MRI can demonstrate ligamentous tears within the sinus tarsi—usually the cervical ligament or interosseous talocalcaneal ligament [32,33]—as well as edema or fibrosis within the surrounding fat. Sinus tarsi syndrome may also occur due to flatfoot deformities and inflammatory arthropathies [34]. Treatment is usually conservative. An arthroscopic subtalar joint synovectomy is an effective treatment when conservative measures fail [35].
Case 8.15 Chondroblastoma with Secondary Aneursymal Bone Cyst
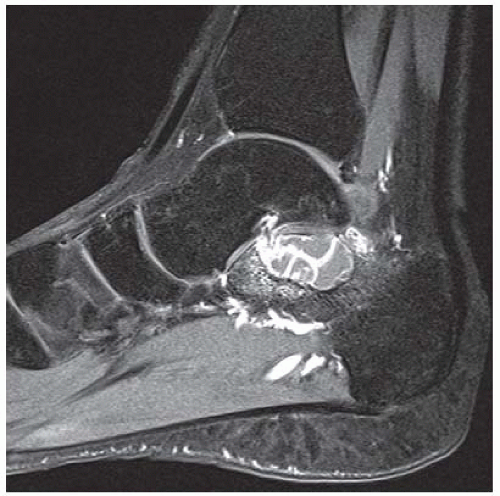
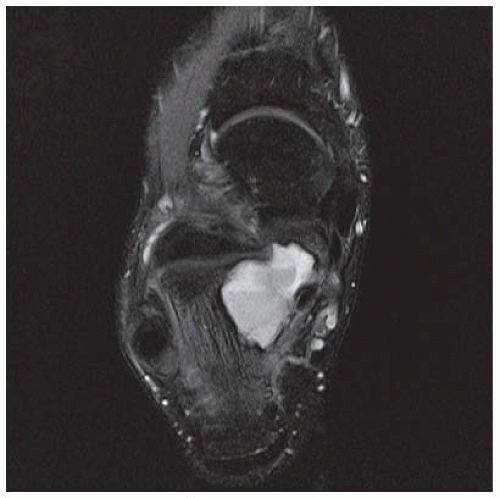
CLINICAL HISTORY A 20-year-old man with foot pain.
FINDINGS
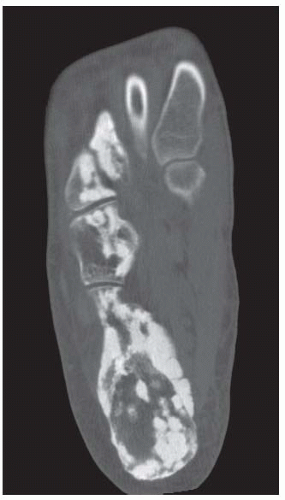
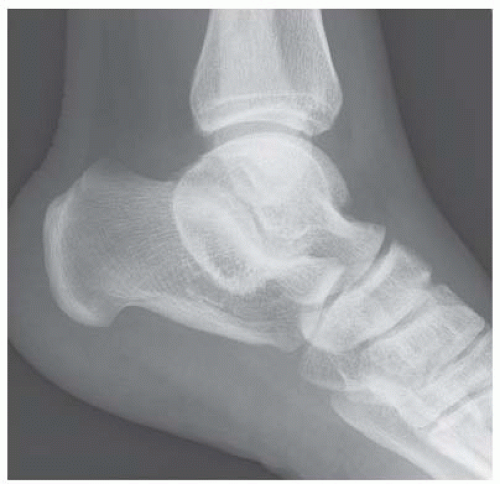
A. Lateral radiograph of the ankle (detailed view) shows a lucent lesion in the calcaneus.
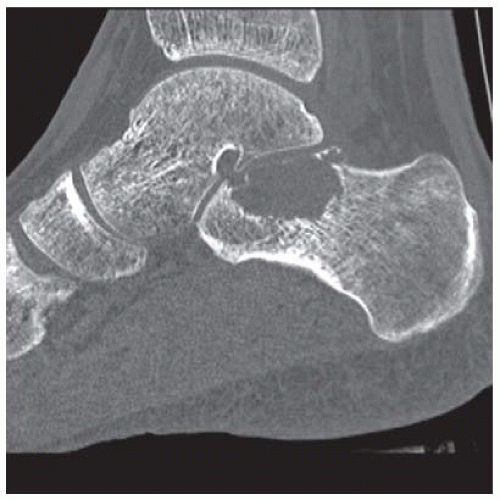
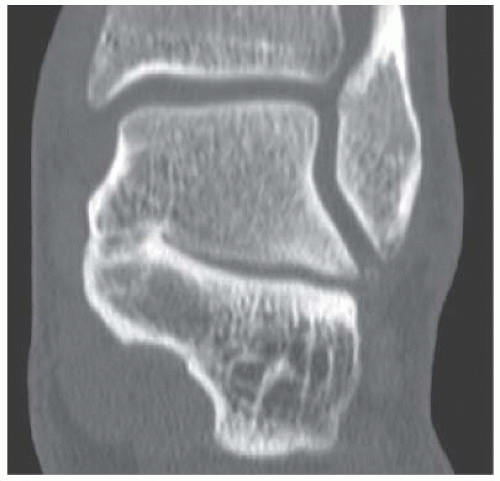
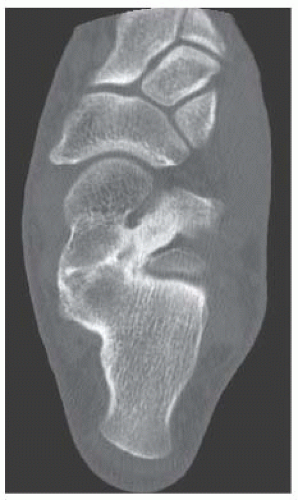
B. Sagittal CT reformation shows lytic lesion in the posterior calcaneus with cortical thinning adjacent to the posterior subtalar joint. There is no sclerotic margin. No matrix mineralization is seen.
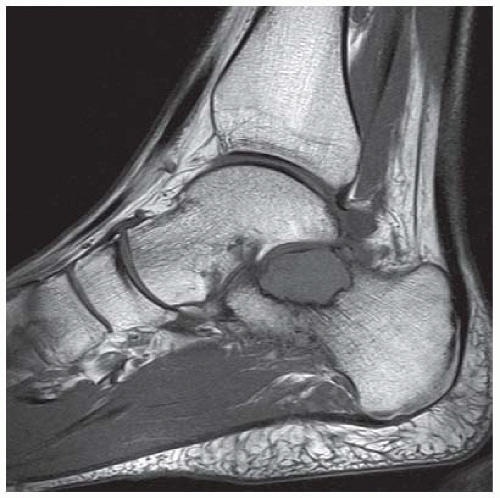
C, D. Sagittal T1-weighted and postcontrast fat-suppressed T1-weighted MRI shows a mass in the calcaneus with peripheral rim and internal nodular enhancement. There are internal thick septa with contrast enhancement, also.
E. Axial T2-weighted fat-suppressed MRI shows cystic lesion with fluid-fluid level and surrounding prominent marrow edema.
DIFFERENTIAL DIAGNOSIS Chondroblastoma, giant cell tumor, aneurysmal bone cyst.
DIAGNOSIS Chondroblastoma with secondary aneursymal bone cyst.
DISCUSSION Chondroblastomas are rare tumors that occur predominantly in patients less than 20 years old. They occur almost exclusively in the epiphyses, but may extend into the diaphyses of long bones. Two-thirds arise in the lower extremities, and half around the knee. When chondroblastomas occur outside the usual age group, they arise in unusual locations.
The radiographic appearance is an ovoid or rounded lucent epiphyseal lesion that is eccentrically or centrally located. The margins are geographic, usually with a thin, reactive bony rim. Scattered mottled calcifications, like those of other cartilage tumors, may be present. They can be secondarily involved by an aneurysmal bone cyst in 10% to 15% of cases. Common preexisting tumors of secondary aneurysmal bone cyst are giant cell tumors, osteoblastomas, and angiomas. Less common underlying processes include fibrous dysplasia, chondroblastoma, chondromyxoid fibroma, unicameral bone cyst, fibrous histiocytoma, eosinophilic granuloma, osteosarcoma, and fibrosarcoma. Secondary involvement of a chondroblastoma by an aneurysmal bone cyst is more likely in patients older than 20 years [31]. Intralesional fluid-fluid levels are common to both chondroblastomas and aneurysmal bone cysts and are therefore not generally helpful for distinguishing the two entities.
Case 8.16 Osteonecrosis
CLINICAL HISTORY A 47-year-old alcoholic with chronic ankle pain but no history of trauma.
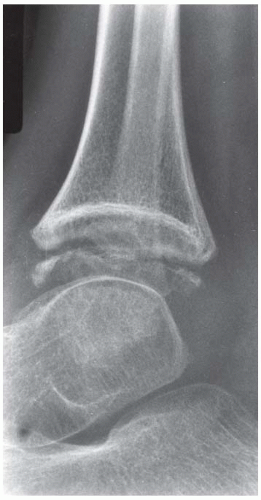
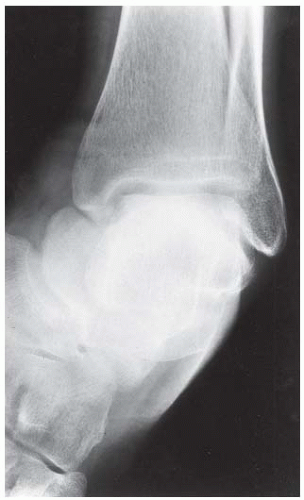
FINDINGS (A-C) Lateral, AP, and mortise radiographs of the right ankle show subchondral lucency, subchondral sclerosis, and flattening of the talar dome.
DIFFERENTIAL DIAGNOSIS Osteochondral fracture, osteonecrosis, osteoarthritis, neuropathic osteoarthropathy.
DIAGNOSIS Osteonecrosis.
DISCUSSION The radiographic findings of linear subchondral lucency, subchondral sclerosis, and collapse of the articular surface are diagnostic of osteonecrosis (avascular necrosis) involving an articular surface. Note the preservation of joint space and normal appearance of the tibial plafond, indicating that the disease process is primary to the talus rather than the joint itself. The opposing joint surface is typically normal in osteonecrosis until late in the disease, when manifestations of secondary degenerative joint disease may be seen. Once revascularization of the necrotic bone has become reestablished, attempts at repair by creeping substitution result in sclerosis as new viable bone is apposed to necrotic bone. Sclerosis represents the earliest radiographic manifestation of osteonecrosis. Resorption of bone may result in a loss of mechanical strength, sometimes leading to subchondral fracture. Subchondral fracture may be evident as linear subchondral lucency—the crescent sign—and it is a highly specific sign of osteonecrosis. The subchondral fracture may even contain nitrogen gas formed in the joint during traction. Continued application of forces across the joint with associated attempts at repair leads to collapse of the articular surface, as in this case. The joint surface may then fragment, and resulting joint incongruity eventually leads to the development of osteoarthritis.
Atraumatic osteonecrosis of the talar dome has numerous causes, including alcoholism, SLE, sickle cell disease, excessive corticosteroids (endogenous or exogenous), pancreatitis, dysbarism, and marrow packing disorders such as Gaucher disease. Some cases have no apparent cause, but the majority of patients with atraumatic osteonecrosis of the talus have osteonecrosis elsewhere, especially the femoral head, and approximately half have bilateral involvement of the tali [36]. Atraumatic osteonecrosis is relatively uncommon, with most cases of osteonecrosis being related to fractures of the talar neck [37].
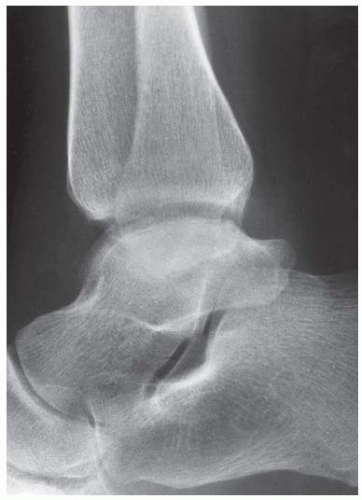
Case 8.17 Subtalar Dislocation
CLINICAL HISTORY A 26-year-old man in a motorcycle accident.
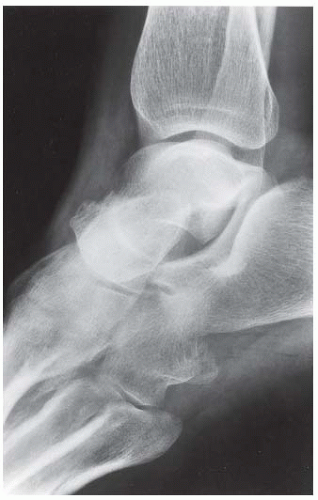
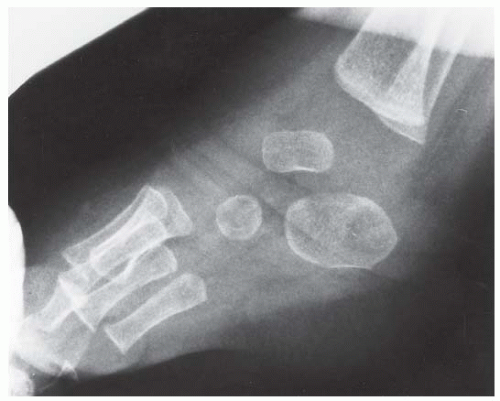
FINDINGS (A) Lateral radiograph of the foot. The talus remains within the ankle mortise, but the relationship of the posterior facets of the talus and calcaneus is abnormally widened and oriented. Easy to overlook, the head of the talus overlaps the navicular, rather than articulating with it. Oblique (B) and AP (C) radiographs of the ankle and foot. The entire midfoot has dislocated medially and inferiorly. Most obvious are the naked articular surfaces of the distal aspect of the talus and the proximal aspect of the navicular.
DIFFERENTIAL DIAGNOSIS None.
DIAGNOSIS Subtalar dislocation.
DISCUSSION Simultaneous dislocation of the talocalcaneal and talonavicular joints is referred to as subtalar dislocation. Of the ligamentous supports about the talus, the talonavicular and talocalcaneal ligaments and capsules are weaker than the calcaneonavicular ligaments and capsule and the ankle ligaments, so the entire midfoot and calcaneus may dislocate as a single unit [38]. Most of these injuries represent high-energy trauma, such as that sustained in motorcycle accidents or falls from a height, but relatively minor stumbling trauma may also cause this injury. Approximately 90% of subtalar dislocations are closed. In approximately 80% of cases, the foot dislocates medial to the talus. Severe open subtalar dislocations occur in major trauma [39], typically motorcycle accidents. Associated injuries include injuries of the tibial nerve, ruptures of the PTT, lacerations of the posterior tibial artery, and articular fractures of the subtalar joint, talonavicular joint, and talar dome. The outcome is often poor, and the risk of osteonecrosis of the talus is significant.
Case 8.14 Sinus Tarsi Syndrome
CLINICAL HISTORY A 18-year-old adolescent boy with painful flatfoot deformity.
FINDINGS
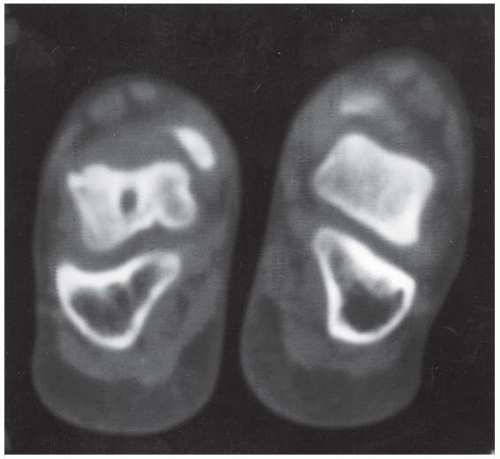
A. Lateral radiograph of the foot shows talocalcaneal coalition, “absent middle facet” sign, and C-sign. The posterior facet of the subtalar joint can be seen.
B. Coronal CT shows a bony coalition between the talus and calcaneus at the sustentaculum tall; the posterior facet is visible and not bridged.
C. Axial CT shows the bony coalition between the calcaneus and the talus.
DIFFERENTIAL DIAGNOSIS Tarsal coalition, surgical fusion.
DIAGNOSIS Tarsal coalition.
DISCUSSION A tarsal coalition is an abnormal bony or fibrous articulation between two tarsal bones. The condition is congenital and appears to result from lack of segmentation rather than fusion of a fully developed joint [40]. Tarsal coalitions are found most commonly as isolated deformities, but they may occur in association with other ipsilateral limb deformities as well as generalized syndromes. Heritable cases of apparently autosomal dominance have been described. Most symptomatic cases are either coalitions between the calcaneus and the navicular (calcaneonavicular), or between the calcaneus and the talus (talocalcaneal). These coalitions may be bilateral in approximately 50% of cases.
A talocalcaneal coalition prevents the rotational and gliding motions at the subtalar joint that normally occur during walking, resulting in a painful flatfoot deformity. The coalition may be fibrous, cartilaginous, or osseous. Typically, the coalition is cartilaginous but becomes ossified as the skeleton matures; with ossification comes rigidity and the onset of symptoms. With calcaneonavicular coalitions, the loss of subtalar motion is partial, and the pain is generally confined to the region of the coalition itself.
Special views and conventional or CT may be necessary to identify the presence and the precise site of coalition. An indirect sign of a coalition between the calcaneus and the talus is talar beaking. A talar beak is a bony spur from the anterior superior aspect of the talus consequent to limited subtalar motion, hypoplasia of the head of the talus, and dorsal subluxation of the navicular bone. A talar beak may occur in any condition that causes abnormal talonavicular motion. The C-sign may be evident on lateral views when a middle facet coalition is present. The dome of the talus forms the top of the C, the coalition forms the middle, and the sustentaculum forms the bottom. The middle facet of the subtalar joint should be visible in normal patients. Thus, when the middle facet is absent, a coalition should be suspected [41]. Coalitions between other adjacent tarsal bones are rare and generally asymptomatic.
Case 8.19 Classic Clubfoot (Talipes Equinovarus)
CLINICAL HISTORY A 1-month-old male infant with foot deformity.
FINDINGS
A. Lateral radiograph of the foot shows that the long axes of the talus and calcaneus are nearly parallel. The longitudinal arch is abnormally high.
B. AP radiograph of the foot shows an abnormally narrow talocalcaneal angle, with severe adduction and supination of the forefoot.
DIFFERENTIAL DIAGNOSIS Clubfoot, vertical talus, metatarsus adductus, skewfoot.
DIAGNOSIS Classic clubfoot (talipes equinovarus).
DISCUSSION Radiographic criteria for the diagnosis of certain congenital foot deformities in infancy are based on lateral and AP views obtained while weight bearing or with dorsiflexion stress simulating weight bearing. In the normal foot, until the age of about 2 years, the long axis of the talus and the long axis of the calcaneus form an angle (talocalcaneal angle) that is about 40 degrees on both lateral and AP radiographs. If one considers the talus to be fixed in the ankle mortise, then abnormal varus (medial) alignment of the calcaneus relative to the talus will reduce the talocalcaneal angle in the AP projection, and abnormal valgus (lateral) alignment will increase it. Because of the geometric and ligamentous linkage between the talus and the calcaneus, the changes in the talocalcaneal angles will be approximately equal on both AP and lateral radiographs. Thus, on AP and lateral radiographs, a small talocalcaneal angle (close to parallel, perhaps 10 degrees or less) is called hindfoot varus, and a large talocalcaneal angle (perhaps 70 degrees or more) is called hindfoot valgus.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree